
23. The thiocarbonyl group |
1445 |
3-alkylidenethiagermiranes 110, which undergo further insertion of germylenes yielding thiadigermetanes 111 as the final products439 (equation 125).
|
R |
R |
|
|
|
|
|
|
|
|
Ge |
|
|
|
|
|
|
|
|
Ph |
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
t-Bu2 C=C=S |
|
|
S |
|
Ph |
Ph |
|
∆ |
R2 Ge: |
t-Bu2 C=C |
|
|
||
|
|
|
Ge |
|
|||||
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
R = Me,Ph |
|
|
|
|
|
|
R2 |
(125) |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
(109) |
|
|
|
|
(110) |
|
R2 Ge: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
t-Bu2 C |
C |
Ge |
|
|
|
|
|
|
|
|
|
|
|
|
Ge
R2
(111)
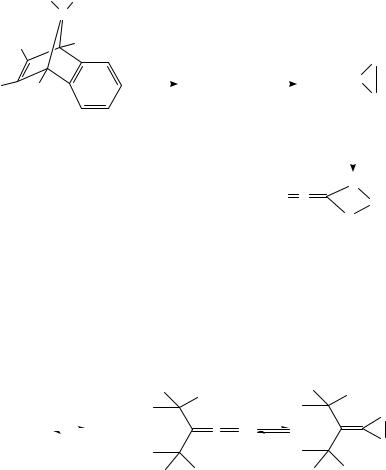
The most common precursors for generating germylenes are the corresponding germanorbornadienes 109 which decompose by thermolysis or photolysis. A related reaction is the stannylene addition to a CDS bond, where the final product is highly dependent upon a fine balance between the steric and electronic factors of the thiocarbonyl compounds involved. Thus, in the case of thioketenes the reaction with stannylene 112 yields first the corresponding thiastannirane 113 which is airand moisture-sensitive. In contrast, on treatment of 112 with thioketones only five-membered dithiastannolanes were isolated440 (equation 126). A compound similar to 113, but more stable, was obtained by addition of (RF)2Sn, where RF is 2,4,6-tris(trifluoromethyl)phenyl, to di-t-butylthioketene441.
|
|
|
|
|
|
Dis2 Sn+ |
• |
SnDis2 |
1/2 |
|
Dis2 Sn |
|
|
||||
|
2 |
|
S |
|||||
|
||||||||
|
||||||||
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
||
|
|
|
|
(112) |
|
(113) |
||
Dis = CH(SiMe3 )2
(126)
2. Thermal [2+2] cycloaddition
In recent years thermal [2C2] cycloadditions have been the subject of several theoretical studies, and in the case of the reaction of substituted thiobenzophenones with phenyllallene mechanistic and kinetic studies were also performed442. Linear free-energy correlations showed that the thione system is more sensitive to electron-donating than to electronwithdrawing substituents. Reactions with heteroatom bonds such as CDB and CDP have also been examined. Thus, thioketones react with borane 114 to give thiaboretanes 115443
1446 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
(equation 127).
|
|
N |
|
Ar |
B Ar |
|
|
|
B N |
+ S C |
(127) |
|
|
S |
|
Ar |
|
|
|
Ar |
(114) |
Ar = 4-ClC6 H4 − |
(115) |
The thio-Wittig reaction between thioaldehyde and phosphorus ylides yields initially a thiaphosphetane, which undergoes cycloreversion to a PDS bond444.
3. [2+3] Cycloaddition
Together with Diels-Alder reactions, [2C 3] cycloadditions are the most common strategies used both in the trapping of unstable thiocarbonyl species and in the synthesis of a wide variety of heterocyclic systems. The first examples of dipolar cycloadditions between diazoalkanes and thioketones date back to 1920445,446, but it was not until Huisgen performed a systematic study, reviewed in 1984447, that thiones reached broad recognition as versatile synthons. In 1989 Huisgen coined the term ‘superdipolarophiles’448 to emphasize the high reactivity of the CDS bond. Thus, 1,3-cycloadditions of diphenyldiazomethane to thioketones are much faster than those to ˛, ˇ-unsaturated compounds and nitriles previously regarded as the fastest dipolarophiles448. The primary cycloadducts, 1,3,4-thiadiazolines 116, extrude N2 and furnish thiiranes 118 via the corresponding thiocarbonyl ylides 117 (equation 128), which are not isolable.
− |
+ |
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Ph2 C |
|
N |
|
N |
|
N |
N |
|
Ph |
R |
|
|
|
Ph |
R |
|||
|
|
|
|
|
|
|
|
|
||||||||||
+ |
|
|
|
|
|
|
|
−N2 |
|
+ C− |
|
|
|
|
|
|||
|
|
|
|
|
Ph |
S |
|
|
Ph |
R |
|
Ph |
R |
|||||
R2 C |
|
|
|
S |
|
R |
|
S |
|
S |
|
|||||||
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
(116) |
R |
|
|
(117) |
|
|
(118) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(128) |
||
A quantitative estimate of the high reactivity of |
thiones is given by the fact that |
|||||||||||||||||
the cycloadditions |
of thiobenzophenone S-methylide |
[formed |
in situ |
by extrusion |
of |
|||||||||||||
N2 from intermediate 116 (R D Ph)] to thiofluorenone and thiobenzophenone proceed 7.8 ð 107 and 1.15 ð 106 times faster than that to methyl propiolate. Even thiofluorenone is 2.4 times more reactive than tetracyanoethylene (TCNE), the superb CDC dipolarophile449.
The regiochemistry of this cycloaddition has been examined by Huisgen and is called ‘thiophilic’: a bond is created between sulfur and the carbon end of the dipole450, although the opposite regiochemistry has been observed sometimes. For instance, diazomethane

23. The thiocarbonyl group |
1447 |
adds in two directions to dialkyl ketones451 and the ratio of the regioisomers depends on the size of the substituents and solvent polarity (equation 129).
|
|
|
|
|
|
|
|
|
|
S |
|
S |
|
|
S + |
− |
+ |
|
|
|
R2 |
|
R2 |
N |
|
R2 C |
|
:N |
|
N |
|
CH2 |
|
N |
N |
+ |
(129) |
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
N |
||
|
|
|
|
|
|
|
|
|
|
(119) |
|
(120) |
Rising polarity of the solvent favors the production of 1,2,3-thiadiazolines 120 whereas increasing steric demand of the thione assists formation of the 1,3,4-isomer 119.
The preference for the 1,2,3-thiadiazoline structure in more polar solvents is explained by the higher dipole moment (ca 5 D as compared to ca 2 D) of the transition structure452. Extensive ab initio calculations on the reaction of diazomethane with thioformaldehyde concluded that both regioisomers should be formed via concerted pathways452, but some semiempirical methods (AM1, MNDO-PM3) suggest that the reaction takes place in a stepwise manner.
The mechanistic picture is more complicated because in reactions of aliphatic thiocarbonyl ylides, generated from sterically hindered thioketones and 2-diazopropane, evidence was found for the formation of zwitterionic intermediates453. These thiocarbonyl ylides are bases and can undergo addition of nucleophiles such as methanol. In the presence of tetrasubstituted acceptor olefins such as TCNE or a variety of dipolarophiles such as dimethyl fumarate, N-phenylmaleimide454, zwitterionic intermediates can close to afford a five-membered thiolane ring or a seven-membered ketene imine ring, and can also undergo rotation to furnish cyclopropane and thione in an intramolecular nucleophilic substitution453.
In equation 128 it is shown that thiocarbonyl ylide 117 may undergo a conrotatory electrocyclic reaction leading to thiirane 118. Thiirane is the smallest sulfur heterocycle and the Munich group has thoroughly studied not only the construction of this system, but also its destruction455, since the elimination of sulfur converts thiiranes into olefins 121 providing an important synthetic application for these molecules (equation 130).
|
|
|
|
Ph |
R |
117 |
|
118 |
PPh3 |
|
|
|
−SPPh3 |
(130) |
|||
|
|
|
|||
|
|
|
|
Ph |
R |
|
|
|
|
|
(121) |
Barton and coworkers exploited this strategy in the preparation of overcrowded ethylenes456: usually the desulfurization of a thiirane is accomplished by one equivalent of tertiary phosphine, mainly triphenylphosphine. However, spontaneous loss of sulfur from thiiranes substituted by aryl or halogen has sporadically been reported. Huisgen has reviewed this subject455 and performed many kinetic studies. He found that the desulfurization step can be accomplished by catalytic thiolates and also by thiobenzophenone or other thioketones, although in this case the reaction is slower (equation 131).
The thiirane donates sulfur to the thioketone, thus furnishing olefin and thiobenzophenone S-sulfide (122) which can be intercepted in 1,3-dipolar cycloadditions to activated acetylenes or thiones455.

1448 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
|
|
|
S + |
S |
|
|
|
|
|
|
|
|
|
Ph2 C |
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
14 days |
|
pentane |
|
(131) |
|||||
|
|
|
|
|
|
|
|
|||||
|
S+ |
|
|
|
|
S+ |
|
|
|
|
||
Ph |
|
Ph |
− |
Ph |
S |
|
||||||
|
S− |
|
|
|
C |
S |
|
|
|
|||
C |
|
|
C |
S |
+ Ph2 C |
|
CH2 |
|||||
|
|
|
||||||||||
Ph |
|
|
|
Ph |
Ph |
|
||||||
|
|
|
|
|
|
|
|
|||||
|
|
|
|
(122) |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|||
In addition to diazoalkanes, a large variety of dipolarophiles have been submitted to cycloaddition with thiocarbonyl compounds226. Reactions often occur at room temperature, are highly regioselective and can be classified after the nature of the 1,3-dipoles according to Sustmann’s classification457, where diphenyldiazomethane belongs to the category of nucleophilic 1,3-dipoles, whereas nitrones (azomethine oxides) are type II dipoles with a nucleophilic electrophilic nature.
Very recently, Huisgen and Sustmann carried out both mechanistic458 and theoretical459 studies of the 1,3-dipolar cycloaddition of several nitrones with aliphatic thioketones finding that, again, thiones are superdipolarophiles vs nitrones458. In the ab initio calculations performed at different levels for the cycloaddition of the parent nitrone to thioformaldehyde, a CT orientation complex was found459. The perturbational analysis shows a strong
HOMOnitrone LUMOthioformaldehyde interaction as the principal reason for the high thione reactivity.
Nitrile oxides have also been used as dipoles, in particular mesitonitrile oxide (MesCNO) which was reacted with different thiones leading to the corresponding oxathiazoles368,460 as shown in equation 132460.
S |
Mes |
MesCNO |
(132) |
S |
|
|
N |
O |
|
Examples of 1,3-dipolar cycloadditions of nitrile sulfides (124) onto thioketones have been reported272 (equation 133). 124 were generated by thermal decomposition of 1,3,4- oxathiazol-2-ones (123) which, in turn, may be prepared bearing a wide variety of substituents R1. Thermolyses were carried out in the presence of the dipolarophile which traps the transient nitrile sulfide, with the formation of 5H-1,4,2-dithiazoles 125. Regioisomeric 1,2,3-dithiazole products were not observed.
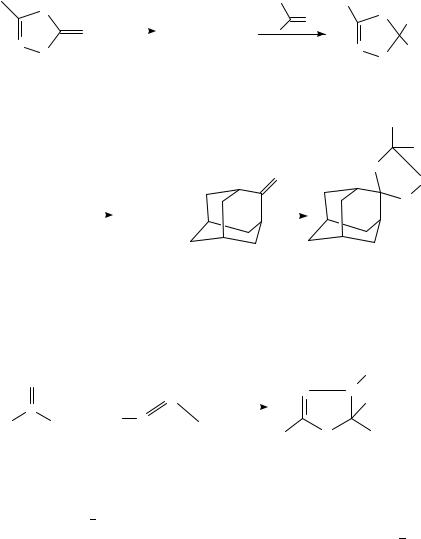
In the case of carbonyl oxides 127, generated by ozonolysis of vinyl ethers 126, the addition to a number of thioketones affords thioozonides 128 in moderate yields461 (equation 134), although sometimes oxidation to sulfines was observed.
|
|
|
|
|
|
23. The thiocarbonyl group |
|
|
1449 |
|||||||||
R1 |
|
|
|
|
|
|
|
|
|
R2 |
|
R1 |
|
|
||||
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
∆ |
|
|
|
|
|
|
|
|
|
S |
S |
R |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
R3 |
|
|||||
|
|
|
O |
|
|
|
|
R1C |
|
N+S− |
|
|
|
|
||||
|
|
|
xylene |
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
N |
|
|
|
|
|
|
|
|
N |
R3 |
||||||||
|
|
S |
−CO2 |
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
S |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(123) |
|
|
|
(124) |
|
|
|
|
|
(125) |
(133) |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R1 R2 C |
|
CHOR3 |
O3 |
|
R1 R2 COO + |
|
|
|
|
|
|
O |
|
|||||
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
(126) |
|
(127) |
|
|
|
|
|
|
|
|
(128) |
(134) |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
We have already mentioned the synthetic versatility of silyl thioketones249 which is confirmed because they react with 1,3-dipoles (nitrile oxides, nitrile imines and nitrile ylides) to give regiospecifically silyl thiaheterocycles462. Equation 135 illustrates the reaction between phenyl trimethylsilyl thioketone and diphenyl nitrilimine.
|
S |
|
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
N |
N Ph |
|
|
|
|
+ |
N |
|
|
|
(135) |
||
|
C |
Ph |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Ph |
|
C |
− |
|
|
|
|||
|
SiMe3 |
|
NPh |
S |
SiMe3 |
|
|||
|
|
|
|
|
|
Ph |
|
||
The reactions with most synthetic applications are those between thioketones and diazo compounds which provide highly conjugated systems after extrusion of nitrogen463. As we mentioned earlier, another application consists of the preparation of sterically overcrowded ethylenes (equation 130): this strategy has been extensively studied by Feringa and collaborators464 467 in the search for chiroptical molecular switches, which are organic materials for optical data storage464. Equation 136 illustrates the thioketone diazo coupling method for the formation of the central double bond in thioxanthene systems467. Hydrazone 129 was oxidized to the corresponding diazo compound 130 and subsequent 1,3-dipolar cycloaddition with thioketone 131 was followed by extrusion of N2 to provide the episulfides 132 and 133. The latter were desulfurized to afford cis and trans isomers which were separated chromatographically467.
Organosilicon compounds have also been reacted with thiocarbonyl ylides to afford a variety of heterocycles468,469 and in Section III.F.5 we pointed out the 1,3-dipolar cycloaddition between in situ generated thioaldehydes and a pyrazolidinium ylide to produce a nuclear analogue of pyrazolidinone antibacterial agents332. Moreover, Vedejs has applied the dipolar cycloaddition between a thione, generated from a Norrish-type II fragmenta-
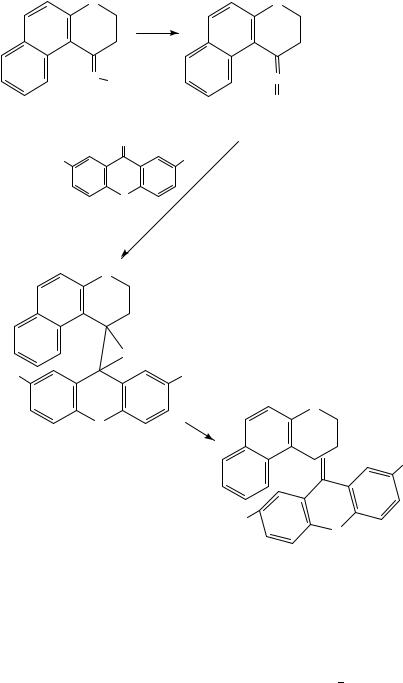
1450 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
S |
S |
|
|
A g2 O |
|
|
−30°C |
|
N |
N+ |
|
|
NH2 |
|
(129) |
N− |
|
(130) |
||
|
||
|
S |
|
Me2 N |
NO2 |
|
|
S |
|
|
(131) |
|
S |
(136) |
|
|
+trans-nitro isomer(133) |
|
|
S |
O2 N |
|
NMe2 |
|
|
S |
|
S |
xylene |
|
|
Cu |
78% |
(132) |
NMe2 |
|
|
O2 N
S
80%
tion, and a nitronate as a key step in the preparation of the natural product methynolide323 (Section III.E.2).
Finally, Isaacs has investigated the effect of high pressure on the 1,3-dipolar cycloaddition of thiones to diazoalkanes, finding that the reaction is strongly accelerated by pressure which is extremely important in the case of sterically hindered substrates470.
4. [2 + 4] Cycloaddition
Diels-Alder chemistry has been reviewed by several authors226,470 472.
23. The thiocarbonyl group |
1451 |
a. Thiocarbonyl compounds as dienophiles. Cycloaddition of a thiocarbonyl compound and a 1,3-diene affords a 5,6-dihydro-2H-thiapyran (equation 137).
|
S |
|
S |
|
|
|
+ |
|
|
|
|
R |
(137) |
|
R |
R′ |
|
||
|
|
|
R′
Virtually all types of thiocarbonyl compounds have been found to react as heterodienophiles. In general, thiocarbonyl compounds are more reactive and versatile dienophiles than the corresponding carbonyl compounds. One major difference between the two types of dienophile is that a dihydrothiapyran formed from a thiocarbonyl group often has a tendency to undergo a thermal retro-Diels-Alder reaction, whereas the corresponding dihydropyran is less prone to do so471. This problem may be partly solved by using high pressure and Isaacs has studied the cycloaddition of thiobenzophenone with several dienes (e.g. isoprene, cyclopentadiene)470. The rate of Diels-Alder reactions is accelerated by high pressure because these reactions have a large negative volume of activation (the TS occupies a smaller volume than the reagents). High pressure can also prevent thermal decomposition which can accompany Diels-Alder reactions.
The competition CDO vs CDS in Diels-Alder reactions is represented in the case of thioxoethanal, generated by FVT, and which only reacts through the CDS bond when trapped with a variety of dienes20,373,377 (equation 138). In the case of cyclic dienes,
endo selectivity was observed and ˛-oxothiones behaved similarly20,379 as well as ˛- oxosulfines20,378.
S
H S
S ∆
 CHO O FVT
CHO O FVT
H O
S
H (138)
CHO
S
H
CHO
Diels-Alder reactions of thioketones are well documented in the literature471, however, since Schaumann published his review1, advances have been recorded in the cycloadditions with thioaldehydes due, mainly, to the development of new synthetic methods (Section III) and techniques (FVT), and therefore the reactions with aldehydes will be highlighted473.
1452 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
Silyl thioketones act as synthetic equivalents of thioaldehydes by protodesilylation with TBAF249,250, as shown in equation 23 (Section III.C.1). A related reaction is the heterocycloaddition of thiones with vinyltrimethylsilylketone, behaving as heterodiene474, to afford 4H-1,3-oxathiins as shown in equation 139.
|
S |
+ |
|
|
|
S |
|
TBA F |
S |
|
|
|
|
|
R |
R |
|||
|
|
|
|
|
|
|
|||
|
R′ |
|
r.t |
|
R′=TMS |
||||
|
|
|
|
|
|
||||
R |
Me3 Si |
O |
Me3 Si |
O |
|
|
O |
||
|
|
|
R′ |
|
|
H |
|||
R = Aryl
R′ = Aryl, TMS
(139)
Very few mechanistic studies have appeared on this subject and Houk group has studied the hetero-Diels-Alder reaction between thioformaldehyde and butadiene475, using ab initio calculations, to show that the reaction is concerted and nearly synchronous. In the case of unsymmetrical dienes, several rules are known to establish the regiochemistry of the cycloadducts1,471.
The most frequently used dienes in the trapping of thioaldehydes have been cyclopentadiene, 2,3-dimethylbutadiene369,370,238 and anthracene351. However, in recent years the applications of thioaldehydes in the synthesis of natural products have been reported mainly by the groups of Kirby, who has reviewed his contributions224, and Vedejs, who generated thioaldehydes by cleavage of phenacyl sulfides and then allowed them to react with electron-rich dienes and ketene acetals, to give, after further transformations, azocine derivatives476. Also, cytochalasans were prepared by using transient thioaldehydes and 2-(tert-butyldimethylsilyloxy)-1,3-butadiene322.
The groups of Kirby224,340 and of Revesz477 have studied the Diels-Alder reactions of thebaine 134 with ethyl thioxoacetate (equation 140) and other thioaldehydes, thus preparing several opiate antagonists.
MeO
|
|
|
O |
|
O |
+ |
EtO2 CCH S |
20 °C |
|
|
|
|||
|
NMe |
|
MeO |
S |
|
|
|
||
|
|
|
|
|
|
|
|
|
CO2 Et |
MeO |
|
|
|
H |
|
|
|
|
|
|
(134) |
|
|
(140) |
|
|
|
|
Stereochemical aspects have been covered by the groups of Turro and Le Noble, who propose hyperconjugation as a crucial factor in face selectivity during cycloaddition of 5-fluoroadamantane-2-thione478.
23. The thiocarbonyl group |
1453 |
b. Thiocarbonyl compounds as dienes. ˛,ˇ-Unsaturated thiocarbonyl compounds (1- thiabutadienes), although usually not stable at room temperature, have been employed in Diels-Alder reactions to prepare thiopyranyl systems226,472. Equation 141 shows the formation of thiometacrolein and its subsequent cycloaddition.
S
|
P4 |
S10 |
|
|
+ |
|
|
∆ |
|
||
|
|
|
|
||
O |
|
S |
S |
S |
|
|
|
|
|
|
S |
(141)
The parent compound, thioacrolein, affords similar cycloadducts which are present in garlic and show potent antithrombotic activity479,480.
Mechanistic studies were performed for simple systems such as 1-thiabutadiene and ethylene481, although dimerization and cycloaddition of thiochalcones have received more attention, including several calculations482 484. Nevertheless, more synthetic utility possesses the uses of Lewis acids as catalysts in hetero-Diels-Alder reactions (equation 142)485,486 involving 1-thiabutadienes.
|
|
|
|
|
Ar2 |
|
RO2 C |
Ar2 |
|
|
|
|
|
|
|
|
|
CO2 R |
|
|
|
|
|
|
|
|
|
|
|
Dimer |
|
|
|
|
|
|
CO2 R |
|
|
|
|
|
|
|
|
Thermal or Lewis acid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ar |
S |
Ar1 |
S |
CO2 R |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
R=Me, (−)-Menthyl |
major |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
Ar2 |
|
|
|
|
|
|
|
|
|
|
CO2 R |
|
|
|
|
|
|
|
Ar1 |
S |
CO2 R |
|
|
|
|
|
|
|
|
minor |
(142) |
Among the Lewis acids investigated, AlCl3 and EtAlCl2 were found to accelerate remarkably the reaction even at low temperatures, the reaction being very slow in the absence of catalyst486, and the asymmetric version has also been reported485.
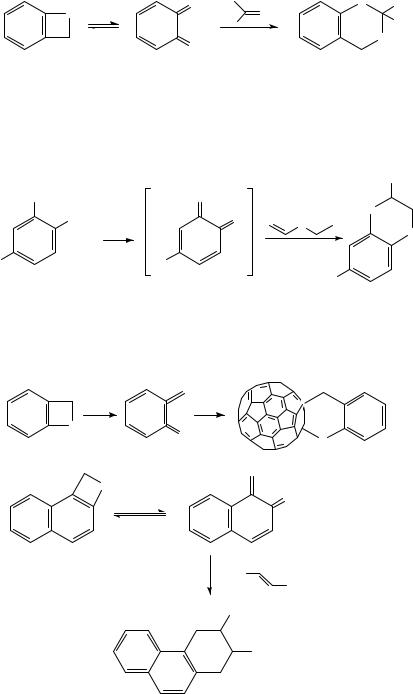
As described earlier o-thioquinonemethide can be generated by several routes353, including thermal ring-opening of benzothietes 72 (Section III.F.3)357 359, and by reaction with carbonyl compounds it yields benzoxathianes 135 as shown in equation 143357.
The reaction of 69 with electron-deficient nitriles furnished 4H-1,3-benzothiazines358 and a wide array of cumulenes were allowed to react with this intermediate359. On the other hand, the reaction with styrenes afforded a variety of benzothiapyrans487.
1454 M. T. Molina, M. Ya´nez,˜ O. Mo,´ R. Notario and J.-L. M. Abboud
|
|
R1 |
S |
R1 |
S |
|
S |
||
∆ |
2 |
O |
|
|
|
R |
|
R2 |
|
|
hν |
|
||
|
|
|
O |
|
|
|
CH2 |
|
|
|
|
|
|
|
(72) |
|
(69) |
(135) |
|
(143)
o-Thioquinonemethide could also be generated from phthalimidesulfenyl chloride by S,N-cleavage (Section III.E.3) and, by reaction with ethyl vinyl ether, gave rise to a variety of 1,4-oxathianes331 (equation 144).
|
|
OR |
OH |
O |
|
SNPhth |
S |
O |
|
||
∆ |
O |
S |
|
||
R |
R |
|
|
R |
(144) |
|
|
Even derivatization of fullerene was achieved by reaction with o-thioquinonemethide 69360 (equation 145). Finally, naphthalene analogs of 69 have been discovered and applied in the preparation of angular systems488 (equation 146).
1 80 ° C C6 0
S |
|
(145) |
S |
|
|
|
S |
|
|
|
|
|
(69) |
|
|
S |
S |
|
80−11 0 °C |
|
|
|
MeO2 C |
(146) |
|
CO2 Me |
CO2 Me
CO2 Me
