
Книги по МРТ КТ на английском языке / MR Imaging in White Matter Diseases of the Brain and Spinal Cord - K Sartor Massimo Filippi Nicola De Stefano Vincent Dou
.pdf
Viral and Non-Viral Infections in Immunocompetent and Immunocompromised Patients |
391 |
27Viral and Non-Viral Infections in Immunocompetent and Immunocompromised Patients
Vincent Dousset
CONTENTS
27.1Introduction 391
27.2Viral Infections 392
27.2.1 |
Viruses in Immunocompetent Patients 392 |
|
27.2.1.1 |
Herpes Viruses |
393 |
27.2.1.2 |
Enterovirus 394 |
|
27.2.1.3 |
West Nile Virus |
394 |
27.2.2 |
Viruses in Immunocompromised Patients 394 |
|
27.2.2.1HIV Encephalitis 394
27.2.2.2Progressive Multifocal Leukoencephalopathy 396
27.2.2.3Cytomegalovirus Infection 396
27.3Prion Diseases 396
27.4Bacterial Infections 398
27.4.1 Bacteria 398
27.4.2 Clinical and Imaging Features 399
27.4.3Bacterial Meningitis 399
27.4.4Subdural Empyema 399
27.4.5Brain Abscesses 400
27.4.6Mycotic Aneurysms 400
27.5Parasitic Infections 401
27.5. 1 |
Cysticercosis (Taenia solium) 402 |
27.5.2 |
Hydatid Cysts (Echinococcus granulosus) 403 |
27.5.3Echinococcus multilocularis 403
27.5.4Toxoplasmosis 405
27.5.5Toxocara canis and Toxocara cati
Infections 405
27.6Mycotic Infections 405
27.6.1Cryptococcosis 407
27.6.2Aspergillosis 407
27.6.3Mucormycosis 407
27.7Granulomatous Infections
and Immunoreactive Diseases 407
27.7.1Granulomatous Infections 407
27.7.2Vasculitis 408
27.7.3 Acute Disseminated Encephalomyelitis 408
References 408
V. Dousset
Professor of Radiology, Université Victor Segalen Bordeaux 2, CHU de Bordeaux –Hôpital Pellegrin, Place Amélie-Raba Léon, 33076 Bordeaux, France
27.1 Introduction
Infectious diseases affecting humans have greatly decreased in the past decades thanks to the antibiotics and the level of hygiene in current life. However, the CNS must be seen as a potential target from many external organisms that have the ability to produce severe diseases with striking symptoms.
Imaging technology, CT and especially MRI, have led to an enhanced ability to characterize infectious processes. MRI techniques such as fast imaging T2weighted images and fluid-attenuated inversion-re- covery (FLAIR) make it possible to depict lesions in the brain, spinal cord, and the meninges. More recently, techniques such as diffusion weighted imaging (DWI) and magnetic resonance spectroscopy, have been applied to inflammatory and infectious lesions, bringing new capabilities for in vivo characterization (Zimmerman 2000; Lai et al. 2002; Cecil and Lenkinski 1998; Burtscher and Holtas 1999). They have an impact on making the positive diagnosis and for the understanding of the disease process.
The appearance of inflammatory lesions is the mirror of multiple factors, including the type of infectious organism, mode of spread, target and host response.
Infections can spread to the CNS in three ways:
Hematogenously, either through the choroid plexus or through the blood–brain barrier (BBB). It is now the most frequent origin of infection in the CNS
Direct spread from adjacent structures, such as the sinuses, nasopharynx, or mastoid air cells
Retrograde axoplasmic flow along cranial or peripheral nerves by some viral agents such as herpes
The imaging features of CNS infections can be classified by the organisms, the location of the lesion and the host response.

392 |
V. Dousset |
The organisms include viruses, mycotic agents, parasites, bacteria and prions (Gray et al. 2004)
The location of the lesions might be one or several of the following: CSF, meninges, parenchyma, arteries, veins, cranial cavities (sinuses, mastoid). It is of importance in an imaging study to look at all these locations
The host response:
1.Immunocompetent patients (child and adults): the response is immunologic and most often symptoms and in vivo images are related to the host response rather than to the infectious agent itself. This means that common imaging features are present for several organisms, making the specific diagnosis somewhat difficult. There is now more evidence for a strong role in the individual genetic background for the development of an organism in the CNS. Not only prions develop in susceptible individuals, but many more organisms are probably infective for some individuals and not others. A transient decrease in the level of immunity may also be responsible for disease development
2.Immunocompromised patients: this group includes several causes such as HIV infection
– which, without treatment leads to deep immunodeficiency – anticancer chemotherapy, diabetes mellitus, long-term steroid administration and, more rarely, congenital immunodeficiency. In these patients, opportunistic agents develop, meaning that these germs might be present in non-immunocompromised people without the ability to develop (Post et al. 1986). HIV has infected more than 60 million people in the world, with 26 million in Africa. In the CNS of HIV-positive patients, some numerous and very specific agents may develop: the HIV virus itself, Toxoplasma gondii, JC virus, tuberculosis, cytomegalovirus (CMV), and Cryptococcus are the most frequent (Gray et al. 2004). CNS type-B lymphoma can also develop. In immunocompromised non-HIV patients, agents such as Candida albicans, mucormycosis or Nocardia may become pathogenic for the CNS
3.Newborns: during birth and for a few weeks after, babies might be affected by infectious agents that are present in the mother’s birth canal: herpes type 2,Listeria monocytogenes,and urinary germs such as E. coli, Proteus, or Candida albicans
4.Embryo and fetus: several agents may develop that can lead to death of the embryo or to fetus CNS malformations. The most frequent agents are Toxoplasma gondii, and CMV, rubella, herpes or HIV viruses (Osborn and Byrd 1991)
5.Finally, the immunologic system may be the origin of CNS manifestations, due to systemic infections of which agents promote a cross-reac- tion with some constitutive proteins of the CNS cells. The organism is usually absent from the CNS. The most sensitive targets are the myelin proteins, leading to acute disseminated encephalomyelitis (ADEM). This includes cross-reac- tion to viruses or bacteria following systemic infection or vaccination. Vasculitis may also be of immunologic origin in response to a systemic organism, leading to cerebral infarct. It is also possible to include in this group some granulomatous diseases, which produce normal immunologic-cell abnormal collection in the CNS, mostly in the meninges, facial cavities or cavernous sinus, e.g., inflammatory pseudotumor and sarcoidosis
We now will describe the infections by the type of organisms affecting the CNS: viruses and prions, bacteria, parasites, fungi, and granulomatous or immunologic reaction. The immunologic state of the host and the location will be discussed in each chapter.
27.2
Viral Infections
The two main features are meningitis and encephalitis. Neurological symptoms will depend on the location of the organism.
Meningitis due to viruses, a frequent infectious disease of immunocompetent hosts, has few imaging manifestations. The role of CT or MRI is questionable
– waiting for imaging modalities may unnecessarily delay the time for lumbar puncture and treatment. Enhancement of meninges is rare.
Viral encephalitis is usually associated with seizure or reduced consciousness or focal symptoms such as motor or sensory deficits. Mild mass effect may be seen during the acute phase of encephalitis. Enhancement is often absent early on during the course of acute encephalitis, with sometimes an enhancement of the adjacent leptomeninges.
27.2.1
Viruses in Immunocompetent Patients
Some viruses may affect both immunocompetent and immunocompromised patients, children, adults

Viral and Non-Viral Infections in Immunocompetent and Immunocompromised Patients |
393 |
or neonates. They belong to the groups of herpes viruses, enteroviruses and arboviruses.
27.2.1.1 Herpes Viruses
Herpes viruses are DNA viruses, and many can cause CNS infections in humans, including herpes simplex viruses 1 and 2, varicella-zoster virus, the EpsteinBarr virus, and cytomegalovirus (CMV) (Tien et al. 1993; Bonthius and Karacay 2002).
Herpes simplex virus 1 is the most common cause of sporadic viral meningoencephalitis. Clinical manifestations include fever, headache, neck stiffness, seizures, focal deficits, and depressed mental state. Because acyclovir therapy is safe, it is recommended that the drug be given on the basis of clinical findings. Encephalitis results from reactivation of latent viral infection of the gasserian (fifth cranial nerve) ganglion. From here,
the infection spreads to the parenchyma. The virus has a predilection for the medial area of the temporal lobes, the frontal lobes and the insular lobes (Fig. 27.1). On CT, low densities are seen on affected areas. There is no enhancement, only the adjacent meninges may show some congestive changes, with very little contrast-agent uptake. On MRI, hyperintensities are encountered in the temporal, frontal or insular areas, and the bilateral nature of the process is frequent. Initially, the infection may appear unilateral on imaging studies, but over time, involvement of the contralateral temporal and frontal lobes will become apparent
Herpes simplex virus 2 is the most common cause of neonatal encephalitis.Infection occurs when the fetus passes through the birth canal of a mother who has genital herpes. Imaging findings reflect rapid brain destruction. Rare observations have been made on adults with extension to the spinal cord
a |
b |
c |
d |
Fig. 27.1a–d Herpes encephalitis. a, b Contrastenhanced CT. Low density of the temporal and insular lobes without focal enhancement. c, d Flair MR imaging. High signal intensities in both insular lobes and in both temporal lobes

394 |
V. Dousset |
Varicella-zoster virus produces two distinct clinical syndromes, chicken pox and herpes zoster. Diffuse encephalitis is a rare complication of chicken pox, but it is more common in adults. It is usually mild. Herpes zoster may lead to an involvement of peripheral and cranial nerves. The affected cranial nerve will appear edematous and swollen, and it will enhance at MR imaging with gadolinium. Herpes zoster may also produce small vessel vasculitis, leading to cerebral infarcts
CMV in adults is seen almost exclusively in immunocompromised patients. However, CMV is the most common cause of congenital encephalitis. It produces massive brain destruction during the first trimester. Infections acquired in the second trimester produce cortical dysplasia
Epstein-Barr virus has been linked to diverse entities, such as Guillain-Barré syndrome, and lymphoma in patients with AIDS. About 5% of the patients with infectious mononucleosis develop an acute, usually self-limited encephalomyelitis. This disorder may be responsible for hyperintensities on T2-weighted images in the deep supratentorial gray matter and central gray matter of the spinal cord. Rapid resolution of the lesions has been reported in this disease
Human herpes virus-6 has been identified as a causeof encephalitisandfebrileseizure(Bonthius and Karacay 2002)
latter, the spinal cord, medulla, pons, mesencephalon, the dentate nucleus of the cerebellum, and occasionally the thalamus may be affected. These structures appear hyperintense on T2-weighted images (Fig. 27.2).
The location in the rhombencephalon and mesencephalon is also the predilection of the bacteria
Listeria monocytogenes.
27.2.1.3
West Nile Virus
This virus has emerged in the United States as a new etiologic pathogen causing encephalitis (Bonthius and Karacay 2002).
27.2.2
Viruses in Immunocompromised Patients
The CNS of immunocompromised patients may be affected by the same viruses that affect immunocompetent patients. Additionally, other viruses only develop in immunocompromised patients (Post et al. 1986; dal Canto 1997).The HIV virus that causes the depletion in immunity may be responsible for encephalitis. Another virus, JC virus, can also cause multifocal encephalitis with destruction of oligodendrocytes.
27.2.1.2 |
27.2.2.1 |
Enterovirus |
HIV Encephalitis |
Enterovirus may be responsible for meningitis and, rarely, for encephalitis (Zimmerman 2000). In the
HIV-1 is the human RNA retrovirus that causes AIDS. The brain is one of the most commonly affected or-
Fig. 27.2 Viral cerebellitis due to enterovirus. Flair MR imaging showing high signal intensities of the cerebellar cortex

Viral and Non-Viral Infections in Immunocompetent and Immunocompromised Patients |
395 |
gans. Almost all patients have the HIV virus in the CNS, and 10–15% may develop a decrease in mental status or dementia.
It has been described that the primary infection to HIV may lead to focal abnormal deep white matter spots recognized as hyperintensities on T2-weighted images (Trotot and Gray 1997).This is a nonspecific sign that should be taken cautiously, since it is very frequent in many other conditions such as aging, high blood pressure, tobacco, and diabetes mellitus. The brain parenchyma is one of the sites of residency of the HIV virus for several years. During the phase of latency, before the patient starts to have AIDS,it has been shown that some degree of atrophy may occur.When immunodepression is becoming stronger, the HIV virus itself causes sub-
acute progressive encephalitis. The organism replicates within multinuclear giant cells and macrophages in the white matter (dal Canto 1997).There is atrophy,water accumulation in the interstitium, slight demyelination, but no inflammatory changes.
The most common finding is generalized atrophy on CT or MR without focal abnormalities (Fig. 27.3a). Some degree of non-atrophic brain shrinkage is caused by systemic effects of the disease. In severe cases, diffuse symmetric hyperintensity is seen in the supratentorial white matter, predominantly in the periventricular region (Fig. 27.3b, c). Mass effect and enhancement are absent (Fig. 27.3d).On T1-weighted images, the white matter appears almost normal or slightly hypointense.
a |
b |
c |
d |
Fig. 27.3a–d HIV encephalitis. a T2 MR imaging. Slight parenchymal atrophy and ventricular enlargement. b T2 MR imaging. Parenchymal atrophy, ventricular enlargement, bilateral and symmetrical deep white matter abnormal high signal intensities. c T2 MR imaging. Severe parenchymal atrophy, severe ventricular enlargement, bilateral and symmetrical deep white matter abnormal high signal intensities. d Gadolinium-enhanced MR imaging. Same patients as c. No gadolinium uptake. No noticeable changes on T1-weighted images

396
27.2.2.2
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is caused by a papovavirus—the JC virus. This virus is ubiquitous in the adult population. It is present in lymph nodes, and it has been suggested that it resides in the kidneys. When a deep immunodepression is present, usually with blood CD4 cells below 100/mm3, the virus infects the myelin-producing oligodendrocytes,which results in severe demyelination with little inflammatory reaction. Patients complain of focal and progressive neurological impairment, with motor or visual function loss or cerebellar syndrome. Demyelination starts at the subcortical white matter, in the U-fibers (Fig. 27.4a–c). Areas of demyelination are seen as hyposignal on T1-weighted images, with a high signal on T2-weighted images and FLAIR images, without mass effect or enhancement (Fig. 27.4d–f) (Post et al. 1986; Dousset et al. 1997). There is always a strong correlation between the symptoms and the location of the abnormalities on MRI.
In the past, PML was inevitably fatal, with death occurring within 6–12 months of the onset. The administration of drugs developed to treat HIV, such as protease inhibitors, can cause, in some cases, a stabilization of the lesions produced by PML, probably by improving the function of the immune system. Additionally, the incidence of PML, around 5% before the development of antiretroviral drugs, has dropped significantly (Gray et al. 2003).
27.2.2.3
Cytomegalovirus Infection
CMVinfectioninimmunocompromisedpatientsleads to ventriculitis and leptomeningitis. Ventriculitis is diagnosed on MRI by the presence of enhancement of ventricle surfaces. The differential diagnosis is subependymal lymphoma.
27.3
Prion Diseases
A group of CNS diseases called transmissible spongiform encephalopathies (TSE) is characterized by spongiform degeneration of neurons in the cortex and subcortical nuclei. It is known to be transmissible since the 1920s, when it was observed that humans in Borneo eating the CNS of defeated warriors
V. Dousset
were affected by a fatal dementia called kuru. Several human and animal diseases produce this distinctive pattern, including kuru, bovine spongiform encephalopathy (mad cow disease) and scrapie (sheep and goat). There are four forms of TSE, according to the way of contamination:
Sporadic Creutzfeldt-Jakob disease (CJD), the most frequent (80%) has a spontaneous and sporadic origin. However, tissues from those patients may transmit the disease to other humans when injected or grafted
Heritable TSE affects families and is known as Gerstmann-Sträussler disease and fatal familial insomnia
Acquired TSE is of medical transmission, when patients are grafted with contaminated tissues from infected donors: blood transfusion, pituitary extracts, dura mater, and corneal transplantation
Variant CJD (vCJD) is believed to affect patients who have eaten meat from bovine spongiform encephalopathy-infected cattle. The epidemic has affected mostly the UK, with more than a hundred deaths and France with at least four deaths since 1996. The epidemia has stopped.
This classification shows the role of an infectious agent that becomes pathogenic in particular genetic settings. Prusiner and others have partially elucidated the origin of TSE (Prusiner 1987). Although still controversial, transmissible agents are likely to be proteins called prions. The normal protein PrPc becomes pathogenic when beta-pleated, thus becoming insoluble and resistant to heat, and is called PrPres. PrPres is capable of inserting itself into the cell membrane of neurons and inducing their own reproduction. It accumulates in the CNS and is neurotoxic. This results in death of groups of neurons within the brain (Gray et al. 2004).
Patients with CJD usually present late in life (>50 years of age) with rapid onset of dementia and myoclonic jerks (Martindale et al. 2003). Most patients are dead within a year of the onset of symptoms.
MRI is becoming a technique of choice for diagnostic orientation. The earliest MRI signs are symmetric basal ganglia and cortical hyperintensities on FLAIR and/or DWI (Fig. 27.5a,b) (Collie et al.2001). In the clinical setting, these MRI signs are quite specific, although not constant. In most cases, the MRI abnormalities of CJD are bilateral and symmetric, but they may be unilateral. Bilateral hyperintensity of the basal ganglia may be seen on top of the basilar artery infarct, deep venous thrombosis, in acute ex-

Viral and Non-Viral Infections in Immunocompetent and Immunocompromised Patients |
397 |
a
Fig. 27.4 a–f. Progressive |
|
|
multifocal leukoencepha- |
|
|
lopathy (PML). a T2 MR |
|
|
imaging. High signal |
|
|
intensity of arcuate fibers |
|
|
under the frontal motor |
|
|
cortex in an HIV-infected |
|
|
man complaining of right- |
|
|
hand paresis. b Flair MR |
|
|
imaging. Same patient as |
|
|
c |
||
in a. The abnormality is |
||
more conspicuous than |
|
|
in part a. The cortex is |
|
|
spared. c T2 MR imaging. |
|
|
Evolution of PML on the |
|
|
same patient as in a and |
|
|
b. Subcortical high signal |
|
|
intensity extending into |
|
|
the frontal white matter. |
|
|
No mass effect. d T1 sagit- |
|
|
tal MR imaging. PML of |
|
|
the occipital lobe of an |
|
|
HIV patient with bilateral |
|
|
visual loss. Atrophy and |
|
|
low signal intensity of |
|
|
occipital-lobe white matter |
|
|
indicating destruction of |
|
|
the axonal fibers. e Flair |
|
|
MR imaging. Bilateral |
|
|
high signal intensity of the |
|
|
subcortical occipital white |
|
|
matter. f Gadolinium-en- |
|
|
hanced MR imaging. No |
|
|
e |
||
uptake of gadolinium |
||
|
b
d
f

398 V. Dousset
a 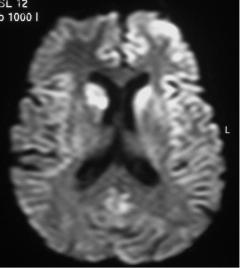 b
b
Fig. 27.5a,b Creutzfeldt-Jakob disease. a Flair MR imaging. Bilateral and symmetrical high signal intensity of the striatum from the basal ganglia. b Diffusion (b=1,000 mm2/s, trace image) MR imaging. High signal intensity of the two caudate nuclei and high signal intensity of the left frontal and insular cortex
position to toxics, and in metabolic disorders such as Wernicke’s encephalopathy. Usually the clinical setting is far different from CJD, making those diagnoses very unlikely.
Variant CJD shows a peculiar MRI sign, with high signal intensity in the pulvinar of the thalami (Collie et al. 2003). This “pulvinar sign” is, however, sometimes seen on sporadic CJD. Lately, atrophy and white matter high signal intensities are present on MRI studies.
Electric encephalograms may reveal the presence of triphasic waves that strongly suggest the disease. This sign is, however, of low sensitivity. CSF might be normal or with increased proteins. The 14-3-3 protein might be suggestively high although not specific. In vCJD, the search for the PrPres protein includes biopsy of lymphoid organs or tonsils.
27.4
Bacterial Infections
Many bacteria may enter the CNS either hematogenously or by contiguity from the paranasal sinuses, the inner and middle ear or through a traumatic or surgical opening in the dura (Zimmerman 2000).The infection may affect one or several compartments of the brain at the same time: subdural (empyema) or CSF spaces (meningitis) and the brain parenchyma
(encephalitis followed by a circumscribed abscess). Arteries, veins and perivascular Virchow-Robin spaces contribute to the spread of the bacteria from one compartment to another. Furthermore, acute or rapidly progressive thromboses of these vessels lead to additional abnormalities. The infection may also reach the surface of the endothelial wall causing the so-called distal mycotic aneurysms (see section 4.6) that have a high risk of rupture.
27.4. 1 Bacteria
Staphylococci and streptococci pneumonia spread to the CNS either by a hematogenous path or via adjacent cranial structures.
Meningococci follow a hematogenous path and produce acute meningitis with high risk of death.
Koch bacilli causing tuberculosis (TB) usually is of hematogenous origin, leading to acute or subacute meningitis, and/or brain abscesses. TB affects many people worldwide, in underdeveloped countries, including patients with AIDS.
Nocardia affects immunocompromised patients
(AIDS or others) and causes many brain abscesses, usually contemporary with chest infection.

Viral and Non-Viral Infections in Immunocompetent and Immunocompromised Patients |
399 |
Listeria monocytogenes may affect newborns or patients eating a high amount of bacteria in contaminated foods. The distribution of Listeria is usually the meninges and/or the rhombencephalon (brain stem and cerebellum), where it produces microabscesses (Maezawa et al. 2002; Gray et al. 2004).
E. coli or Proteus, bacteria of urinary origin, can cause brain abscesses in neonates.
Tropheryma whippelii causing Whipple disease is a rare infection, usually, but not constantly, encountered in patients with digestive malabsorption. It may appear as small lesions disseminated throughout the entire CNS with a predilection for gray matter (cortex, nuclei) (Gray et al. 2004).
Treponema pallidum causing syphilis is becoming a very rare cause of CNS infection. It produces mostly chronic meningitis, and, in a few cases, granulomatous reactions have been described along the cranial nerves.
Borrelia burgdorferi transmitted to humans by insect bytes causes Lyme disease. Involvement of spinal or cranial nerve roots is frequent. An immunological process leading to the involvement of the white matter resembling MS is much rarer.
27.4.2
Clinical and Imaging Features
Systemic signs of infection (e.g., fever and leukocytosis) may be present. Signs of CNS contamination include one or several of the following: neck stiffness and photophobia when meninges are affected, seizures and focal deficit or cerebellar signs when the parenchyma is involved.
Imaging features reflect the host reaction and are of variable appearance according to the type and location of the bacteria. Imaging techniques such as FLAIR and DWI, including the calculation of apparent diffusion coefficient (ADC) maps, are now used routinely in the indication of inflammatory CNS diseases. On DWI, purulent material is usually hyperintense and the decreased ADC shows the restriction of water motion (Desprechins et al. 1999; Lai et al. 2002). ADC helps in differentiating brain abscesses from CNS tumors. Indeed, necrotic debris from CNS tumors such as glioblastoma or metastases has variable and heterogeneous intensities and usually an increased ADC. There are, of course, some over-
laps, especially in parasitic toxoplasmic abscesses or in punctured bacterial abscesses that may show increased ADC. MR spectroscopy, which is less routinely used, reveals the presence of amino acids from extracellular proteolysis and bacterial metabolism (fermentation products), including succinate, acetate, leucine, valine, and alanine, which are not seen in necrotic neoplasms (Lai et al. 2002; Cecil and
Lenkinski 1998; Burtscher and Holtas 1999).
27.4.3
Bacterial Meningitis
The diagnosis is confirmed with lumbar puncture, and imaging does not play a primary role in the detection or treatment of this disorder. It is recommended to treat the patients as early as possible, without waiting for unnecessary imaging modalities. CT may be used to exclude increased intracranial pressure prior to lumbar puncture—only when there are clinical doubts. Meningitis without parenchyma involvement has a normal appearance on CT and MR T2-weighted images.
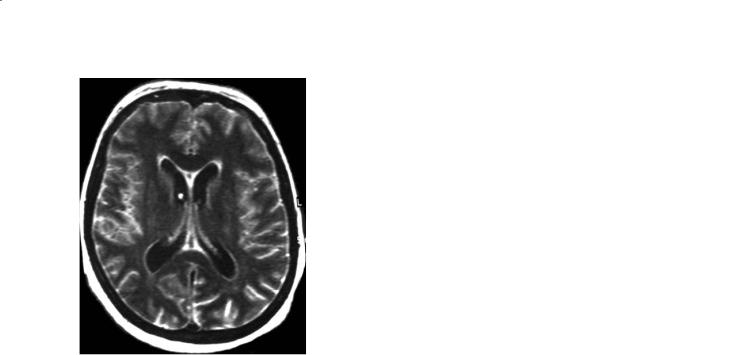
FLAIR imaging might be helpful in the diagnosis of meningitis, if the clinical presentation is not straightforward. It shows diffuse subarachnoid hyperintensity, while the CSF in the ventricles is dark (Fig. 27.6). However, there are three differential diagnoses when the subarachnoid space appears bright on Flair: (1) a CSF flow artifact, (2) a subarachnoid hemorrhage, (3) a hyperoxygenation such as during 100% oxygen supplementation for anesthesia or during a status epilepticus.
In bacterial meningitis, contrast-agent enhancement in the CSF space can be present, although it is unusual when the parenchyma is not involved. Tuberculous meningitis may be seen as an enhancement in the cisterna and along the sylvian fissures. CSF space enhancement may also evoke granulomatous diseases.
27.4.4
Subdural Empyema
Subdural empyema may be the result of direct spread of infection from the paranasal sinuses or the middle ear, or it can be of hematogenous origin or follow meningitis or cerebritis, through the venous structures. Subdural empyema produces an acute progressive syndrome characterized by fever and rapid development of neurologic abnormalities
400 |
V. Dousset |
Fig. 27.6 Viral meningitis. Flair MR imaging. High signal intensity of the sub-arachnoid CSF space
(e.g., seizure and hemiparesis) (Rich et al. 2000). A complete imaging study of the brain and cranial structures is compelling in cases of subdural empyema. Subdural empyema produces brain complications by retrograde venous thrombosis that leads to cortical venous stasis with marked cortical swelling and brain abscesses.
Subdural empyema can be difficult to detect, particularly on unenhanced CT images (Fig. 27.7). The collection is typically narrow. There is disproportionate mass effect,with diffuse swelling of the hemisphere adjacent to the collection (Zimmerman 2000). The cortex may appear thickened because of venous stasis. There might be evidence of sinusitis or mastoiditis.
On MR images, the subdural collection is more conspicuous, particularly on FLAIR images, where it appears hyperintense to adjacent brain. On DWI, the content may appear bright, and the ADC values low (Fig. 27.7d). MR spectroscopy reveals the presence of amino acids. Contrast-enhanced CT and MR images reveal thin enhancement of the deep and superficial membranes of the subdural empyema (Fig. 27.7b, c).
27.4.5
Brain Abscesses
An abscess is the result of the host defense against bacteria, which initially produce a diffuse cerebritis or encephalitis. Macrophages produce a true collagenous capsule that marks the passage from cerebritis to the abscess phase. On CT, the capsule may appear
with a slight increased density. Contrast enhancement with iodine contrast agents takes a regular ring appearance (Fig. 27.7b). The capsule made of fibrin and collagen has a typical appearance on MRI: low signal intensity on T2-weighted images and FLAIR, and ring enhancement with gadolinium (Fig. 27.7c). Additionally, on FLAIR and T2-weighted images, vasogenic edema appears as a high signal intensity infiltrating the white matter (Zimmerman and
Weingarten 1991).
ThecentralnecroticregionishyperintenseonFLAIR images, and isointense to CSF on T2-weighted images. On diffusion imaging the center appears bright, which may be due to “T2 shine-through” effects. On ADC maps, the central necrotic material is hypointense, due to the restriction of water motion, because the water molecules move slowly in the dense abscess content, made of thick proteins and mucus (Fig. 27.7d). In at least two circumstances, the ADC may be increased: in toxoplasmic abscesses and in bacterial abscesses that have been punctured. Nevertheless, the decreased ADC values help to differentiate from brain necrotic tumors or metastases, which have increased ADC values. In brain abscesses, MR spectroscopy with long TR sequences reveals the presence of amino acids that are the proteolytic breakdown and fermentation products unique to bacterial infection. Enhancement will persist for up to 8 months.
A peculiar feature of brain abscesses is the miliary (Fig. 27.8). It may happen following the hematogenous spread of TB or Nocardia. Innumerable, small abscesses are present in the parenchyma.
27.4.6
Mycotic Aneurysms
Intracranial infectious aneurysms are important features that are not rare. They usually occur in patients with staphylococcal endocarditis and are called “mycotic” aneurysms (Phuong et al. 2002). They also develop in intravenous drug abusers (Tunkel and Pradhan 2002). Their imaging presentation is usually a small mass in the subarachnoid space near the cortex with strong enhancement. They may rupture, leading to subarachnoid hemorrhage with high risk of death. They also can be revealed by focal infarcts or seizures (Fig. 27.9). Note that stroke may occur without infective aneurysms in patients with valve endocarditis (Anderson et al. 2003). Non-rup- tured aneurysms may disappear under antibiotics. Ruptured and sometimes non-ruptured aneurysms need endovascular treatment or surgical clipping.
