
новая папка / 450-550 operativ
.pdf
462 |
SRB’s Surgical Operations |
can be reduced) with 2 gm calcium supplement. Monitoring is done by evaluating serum calcium, albumin, magnesium, and phosphorus and bone speci c alkaline phosphatase.
Thyrotoxic Storm/Crisis
Thyrotoxic storm is an unusual complication of thyroid surgery. is condition may result from manipulation of the thyroid gland during surgery in patients with hyperthyroidism. It can develop preoperatively, intraoperatively, or postoperatively. It is observed in thyrotoxic patients who are inadequately prepared/controlled; it begins due to surgery (thyroidectomy) or anyother stress or operations other than thyroidectomy. yrotoxic storm is potentially lethal and must be dealt with utmost care. It can be seen in toxic nodule or toxic nodular goitre or more commonly in Grave’s disease. Mortality is more than 50%. ere is exacerbation of hyperthyroidism with
decompensation of organ systems.
Features
Features of thyrotoxic storm in the anaesthetised patient include evidence of increased sympathetic output, such as tachycardia and hyperthermia. In an awake postoperative patient features are nausea, tremor, severe dehydration, circulatory collapse, hypotension, hyperpyrexia, out of proportion tachycardia, cardiac arrhythmias/ failure and altered mental status. Cardiac arrest may also occur. If treatment is not adequate, the patient may progress to coma. Prevention is by restoring the thyroid status as close to euthyroid as possible. Morbidity and mortality rates in adequately prepared patient are low.
Intraoperative Management
e rst step in managing a thyrotoxic crisis during thyroidectomy is to stop the procedure. Intravenous beta-blockers, PTU, sodium iodine, and steroids areadministered tocontrol sympathetic activity, therelease of thyroid hormone and hyperthermia. Cooling blankets and cooled intravenous uids are used to reduce the patient’s body temperature. Oxygen demand increases dramatically during a thyroid storm.
Treatment of thyrotoxic crisis postoperatively
•Injection hydrocortisone 500–1000 mg intravenously.
•Proper fluid therapy to maintain hydration, blood pressure.
•Control of hyperpyrexiaby total body sponging, cooling blankets, cooled intravenous fluids, steroids (dexamethasone), antipyretic injections.
•Sedation using chlorpromazine.
•Digoxin and diuretic therapy with monitoring ofblood pressure.
•Intravenous heparin in case ofatrial fibrillation.
•Beta blockers – oral propranolol – 80mg 4th to 6th hourly (through nasogastric tube if needed); or oral diltiazem. Heart rate should be kept below 100/minute. Often to achieve this intravenous esmololmay be needed. Refractory cases require reserpine therapy.
•Antithyroid drugs – orally. Propylthiouracil or methimazole or carbimazole is given. Lugol’s iodine is given4 hours after propylthiouracil. Injectionpropranolol also can be given.
•Plasmapheresis and charcoal plasma perfusion or exchange perfusion is used if patient does not respond in 48hours.
•Intravenous injection of sodium iodide 1 gram is often used.
•ICU care, oxygen, ventilator support, cardiac monitoring; electrolyte/ blood gas/liver and renal function/thyroid hormone estimationat regular intervals is needed.
•Preventionofsecondepisodeofcrisisbycontinuingthetherapyisneeded.
External Laryngeal Nerve Injury
The superior laryngeal nerve has two branches—internal and external. e internal branch provides sensory innervation to the larynx. It enters the larynx through the thyrohyoid membrane and not at risk during thyroidectomy. The external branch supplies cricothyroid muscle after passing through cricothyroid space of Reeves and is at risk during thyroidectomy. This muscle causes elongation of the vocal folds (tensor). Injury to this branch causes inability to lengthen the vocal fold and creates a high-pitched sound.
e external nerve is probably the nerve most commonly injured in thyroid surgery (0-25%).
Features
Most patients do not notice any change. Patient may present with mild hoarseness or decreased vocal stamina. However, for a singer or a person who professionally relies on his or her voice (teachers), paralysis of this nerve may threaten his or her career. The most damaging consequence is loss of the upper register.
Diagnosing an external laryngeal nerve injury with indirect or beroptic laryngoscopy is di cult. Posterior glottic rotation towards the paretic side and bowing of the vocal fold on the weak side may be noted. e a ected vocal fold may be lower than the normal vocal fold. Use of videostroboscopy and laryngeal EMG will help to diagnose ELN paralysis. Videostroboscopy demonstrates an asymmetric, mucosal traveling wave. EMG demonstrates denervation of cricothyroid muscle.
Prevention and Treatment
e external branch of SLN travels inferiorly along the lateral surface of the inferior constrictor until it terminates at the cricothyroid muscle. is branch is intimately related to the superior thyroid artery, though its exact relation to the artery varies. Study suggests that the nerve may cross the superior thyroid artery more than 1 cm above the upper pole of the thyroid gland (42%), less than 1 cm above the upper pole (30%), or under the upper pole (14%). In some people, the nerve runs dorsal to the artery and crosses only its terminal branches after the artery has rami ed (14%). e critical area, 1.5-2 cm from the thyroid capsule is described. In this area, the ELN is most intimately involved with the branches of the superior thyroid artery.
Most surgeons agree that identifying the ELN, in contrast to the RLN, is unnecessary even though by principle it is ideal and must. Instead the terminal branches of the superior thyroid artery are ligated as close to the thyroid capsule as possible to avoid nerve damage. Direct trauma to the cricothyroid muscle can cause brosis and poor muscle function, which may result in a presentation similar to that of a patient with an injury to the ELN, even when the nerve is preserved. erefore careful dissection near this muscle is advised to avoid damage due to electrocautery. Treatment for ELN palsy is speech therapy.
Hypothyroidism
Systemic thyroid hormone levels are low in hypothyroidism. It may be primary due to thyroidectomy/radioactive iodine ablation/primary thyroid diseases or may besecondary dueto hypopituitarism or tertiary due to hypothalamic diseases.

Presentation
Untreated hypothyroidism causes symptoms such as cold intolerance, fatigue, constipation, and muscle cramping, brittle hair, dry skin, sluggishness, hypothermia and weight gain. CVS manifestations are important which present as bradycardia, hypotension and conduction problems and pericardial e usion. Hypothyroidism secondary to thyroid surgery should never be left untreated long enough to elicit signs and symptoms of myxoedema (e.g. hair loss, large tongue, cardiomegaly). One should suspect, diagnose and promptly treat postoperative hypothyroidism.
Hypothyroidism will worsen the situation of critically ill patient causing severe pulmonary dysfunction, pleural effusion and hypothermia.
Renal insu ciency can occur in hypothyroidism and so renal function tests should be done.
Evaluation
The most useful laboratory test for detecting or monitoring hypothyroidism in a patient whohas undergone thyroidectomy is the measurement of thyrotropin, thyroid-stimulating hormone (TSH) levels. Total T4 and T3 levels may be useful to ne-tune the dosing of levothyroxine, but may be unhelpful in the typical postoperative patient. In primary type T4, free T4, free T3 are low; but TSH level is high. In secondary type, free T4 index, free T3 and TSH are all low.
Adrenal insu ciency is associated with secondary type.
Features of myxoedema |
Precipitated |
Treatment |
coma |
by |
|
Hypothermia– skin is deadly |
Cold |
Drug of choice – IV T3 10 µg |
cold like atoad. |
infection, |
Levothyronine 300 µg IV; |
Hypotension |
trauma. |
later 1000 µg orally – large |
Hyponatraemia |
|
dose. |
Hypoventilation |
|
Glucocorticoids – IV |
Hypoglycaemia |
|
Antibiotics |
Bradycardia |
|
Slow warming |
Loss of tendon reflexes |
|
Fluid and electrolyte therapy |
Cardiopulmonary collapse |
|
Monitoring; ICU care; |
Coma |
|
ventilator |
Prevention
Hypothyroidism is an expected sequelae of total thyroidectomy. In goitre surgery, the extent of thyroidectomy is controversial. e main goal of surgery is to prevent recurrent hyperthyroidism because recurrent hyperthyroidism after surgery is more di cult to manage than permanent hypothyroidism. Because of this fact, the present authors recommend total thyroidectomy in this setting.
Treatment
Levothyroxine (about 1.7 mcg/kg/d) 100-150 µg/daily (single dose, morning) is started. eir thyrotropin level is checked in 4-6 weeks to adjust the dosage. Patients who are to receive postoperative radioiodine therapy with scanning must stop taking levothyroxine before the procedure. In cardiac patients initially 50 µg/day is started; later gradually increased to required dose.
In myxoedema coma, IV hydrocortisone, IV levothyroxine, IV levothyronine is started.
Chapter 15 Surgery for Thyroid and Parathyroid |
463 |
Types of hypothyroidism
Primary– Thyroidectomy or thyroid diseases like thyroiditis – free T4, T4, and free T3 are low; TSH is high.
Secondary – Hypopituitarism– TSH, free T3 are low. Tertiary – Hypothalamic diseases.
Presentations
Asymptomatic.
Chronic hypothyroidism status.
Myxoedema coma –Acute presentation with loss of deep tendon refluxes, respiratory problem, cardiac irregularities, coma with50% mortality.
Causes
•Agenesis or dysgenesis.
•Enzyme deficiency.
•Iodine deficiency.
•Hashimoto`s thyroiditis.
•Antithyroid drugs.
•Radioiodine.
•Drugs: Lithium, Amiodarone.
•After thyroidectomy.
Clinical features
•General: Tiredeness, weight gain, cold intolerance, goitre, hyperlipidaemia.
•Cardiovascular: Bradycardia, angina, cardiac failure, pericardialeffusion.
•Haematological: Anaemia.
•Dermatological: Dry skin, vitiligo, alopecia, erythema.
•Reproductive: Infertility, menorrhagia, galactorrhoea.
•Gastrointestinal: Constipation, ileus.
•Developmental: Growth and mental retardation, delayed puberty.
•Other features: Carpal tunnel syndrome, myalgia, hoarseness, deafness, ataxia, depression, psychosis (myxoedema madness).
Infection
Infection is rare due to good aseptic methods. Perioperative antibiotics are used in many centres. Bleeding from vascular thyroids, haematoma, infected suture material are the causes of infection. In large malignant thyroid during thyroidectomy injury may occur to oesophagus which causes severe localised sepsis in the neck. Gastrogra n study, CT scan/MRI may reveal the site and extent of the injury. Pus culture, starting higher generation antibiotics, nasogastric tube insertion, and endoscopic identi cation of the injured area are needed management in such rare patients. Usually they recover but may need to keep the nasogastric tube (feeding can be done through the tube) for few weeks.
Recurrent Thyrotoxicosis
Recurrent thyrotoxicosis is di cult to manage. It is more common with subtotal thyroidectomy (4-15%) than with total thyroidectomy. So at present, total thyroidectomy is commonly done for toxic thyroid. Management is proper anatomical assessment of the residual thyroid tissue by using radioisotope scan, MRI neck and radioactive iodine ablation of residual thyroid tissue if patient’s age is more than 45 years or surgical reexploration and complete removal of residual thyroid tissue on both sides. It carries higher risk of injury to recurrent laryngeal nerve and parathyroids. Meticulous dissection, adequate exposure and proper retraction is needed.
Minor Complications
Small seromas can be left as such to resorb. Large seroma may require repeated aspirations. Wound infection and poor scar are other complications.

464 |
SRB’s Surgical Operations |
|
|
|
|
Minimally Invasive Video-assisted |
|
||
|
Thyroidectomy |
|
|
|
|
Minimally Invasive Video-assisted |
yroidectomy (MIVAT)is becoming |
||
|
popular for small nodules and gland without thyroiditis. But it is costly. |
|||
|
e development of videolaparoscopic surgery in the last decade |
|||
|
has allowed several operations to be performed with minimally |
|||
|
invasive techniques. After the |
rst parathyroidectomy procedure |
||
|
performed endoscopically in 1996, this minimally invasive approach |
|||
|
was applied to thyroid surgery. MIVAT was described in 1998. |
e |
||
|
technique was described using a central access with a 1.5 cm incision |
|||
|
and external retraction. Postoperative morbidity rates in patients seem |
|||
|
tobe equivalentto those of patients who have undergone conventional |
|||
|
surgery. |
|
|
|
|
Chao et al (2004) prospectively compared video-assisted thyroid |
|||
|
lobectomy and conventional lobectomy in 116 patients with thyroid |
|||
|
nodules. Transient RLN palsy occurred in 5 (8.5%) patients who |
|||
|
underwent conventional surgery versus 3 (5.8%) patients in the |
|||
|
video-assisted group; the di erence was not signi cant. Patients in |
|||
|
both groups were discharged home on the second postoperative day. |
|||
|
In a 5-year study, Miccoli et al (2004) selected 579 patients to |
|||
|
undergo MIVAT. e operation consisted of total thyroidectomy in |
|||
|
312 patients and lobectomy in 267. |
e mean operative time was 41 |
||
|
minutes (range, 15-120 min) for lobectomy and 51.6 minutes (range, |
|||
|
30-140 min) for total thyroidectomy. |
e postoperative hospital stay |
||
|
was 24 hours (overnight discharge) for all patients. Complications |
|||
|
included postoperative bleeding (0.1%), recurrent nerve palsy (1.3%), |
|||
|
and de nitive hypoparathyroidism (0.2%). Some have suggested |
|||
|
that pain following VAT is less when compared to conventional |
|||
|
thyroidectomy because of limited dissection, retraction, and injury |
|||
|
to tissues. |
|
|
|
|
MIVAT provides endoscopic magnification of nerves and |
|||
|
vessels,potentiallydecreasing the risk of injury to these structures. |
e |
||
endoscopic technique cannot be used in nodules larger than 3.5cm because the specimen is too large to retrieve through the incision. In addition, this technique is not ideal for removing carcinomas in which an intact capsule is desired for oncologic reasons and for accurate histological assessment.
In summary, if the general principles of conventional thyroid surgery are followed, video assistance may help in identifying structures and in minimising the incision. Experienced surgeons may consider using this technique when the equipment and additional surgical sta is available for patients with small follicular nodules/ adenomas, or well-differentiated papillary carcinoma, or when prophylactic thyroidectomy is being performed in oncologically needed patients.
 PARATHYROID SURGERY
PARATHYROID SURGERY
Surgical Anatomy of Parathyroid
Two pairs of parathyroids are located within the false capsule and posterior border of thyroid gland. Superior parathyroids develop from endoderm of 4th pharyngeal pouch and called as parathyroid IV; inferior parathyroids are called as parathyroid III (3rd pouch). Each parathyroid is lentiform/pea sized in shape with 6 × 4 ×2 mm in size weighing 50 mg and red brown in colour. Glands lie in close relation to anastomotic branch of superior and inferior parathyroid arteries. Superior usually lies in constant position at the middle of posterior border of the thyroid, behind recurrent laryngeal nerve. Inferior lies
in variable positions – within the thyroid capsule below the inferior thyroid artery/outside the thyroid capsule above the inferior thyroid artery/within the thyroid substance in posterior border. Inferior is usually in front of the recurrent laryngeal nerve. Inferior parathyroid and thymus develop from 3rd pharyngeal pouch and so can also be occasionally located in mediastinum. Parathyroids receive their blood supply from inferior thyroid artery (80%) and anastomotic artery. Single end artery is the common method of supply (Fig. 15-45).
Glands (chief cells) secrete parathormone (PTH) which controls the calcium metabolism.
PTH
•Increases absorption ofthe calciumfrom the gut.
•Mobilises calcium fromthe bone.
•Increases the calcium reabsorption from the renal tubules.
•Halflife is 4 minutes.
Indications for Parathyroidectomy
Primary hyperparathyroidism – Criteria (2002) for surgical intervention are –raise in serum calcium level more than 1 mg/dl of upper limit of the normal calcium range; 24 hour urinary calcium if more than 400 mg; creatinine clearance whenreduced more than 30%; bone density greater than two standard deviations below peak bone mass in lumbar spine/hip/lower end of radius; age below 50 years; when medical therapy is not possible.
Common cause for primary HPT is single benign parathyroid adenoma (85%). 95% of such adenomas are single in one gland. 5% cases are having double adenomas each one in two glands. Proliferation causing hyperplasia involving all four glands (15%) also causes primary HPT. is type may be associated with MEN syndrome type I and IIA. Slow growing invasive parathyroid carcinoma accounts for 1% of primary HPT.
Four dimensional CT, PET scan, Technetium 99m sestamibi scintigraphy are the present methods of evaluation in these patients. On table methylene blue injection (5 mg/kg over an hour infusion) will make only the parathyroid gland blue to identify it.
Secondary HPT is an indication for removal of all four glands with autotransplantation of parathyroid only in severe cases or with renal osteodystrophy.
Fig. 15-45: Anatomy of parathyroid glands.

Indications for parathyroidectomy
•Severe symptoms
•Young age group
•Markedly reduced bone density
•Serum calcium more than 11mg %
•Urinary calculi
•Neuromuscular presentations
•Urinary calcium more than 400 mg/24 hours
Preoperative Preparation
Vocal cords should be assessed by preoperative indirect laryngoscopy.
High calcium levels preoperatively may require treatment with hydration; diuresis; steroids (prednisolone 20 mg TID for 5 days before surgery); 100 mmol phosphate infusion in 6 hours; 200 units calcitonin subcutaneous injectionfor 5 days twice daily beforesurgery; diphosphanate – etiodronate disodium 7.5 mg/kg daily as slow IV infusion for 3 days; mithramycin 25 µg/kg as single dose.
Anaesthesia and Position
General anaesthesia is used with neck hyperextension by placing rolled sheet under the shoulder blades. Head is placed on the head
– ring; head end of the table is raised to semi–erect position (SemiFowler position).
Incision and Dissection
It is same as for thyroidectomy. Flaps are raised in similar way. Strap muscles are separated after opening the deep fascia in the midline. Thyroid gland is mobilized to identify the parathyroid adenoma. Parathyroid having adenoma is mobilized which is close to recurrent laryngeal nerve. End artery of the parathyroid is identified and ligated. Adenoma is separated from adjacent thyroid tissue using gauze dissection. Either on table venous sampling for PTH assay is done or venous sample from cubital vein is done for PTH assay.
Parathyroid may be con rmed by frozen section biopsy or on table aspiration of parathyroid tissue which is analyzed for PTH assay which will be more than 1500 pg/ml (con rms removed tissue as parathyroid).
Total parathyroidectomy is done for parathyroid hyperplasia by removing all four glands and ⅓ of one gland is autotransplanted into the forearm muscle (brachioradialis) or sternocleidomastoid muscle with marker stitch. Transplanting gland is sliced into 1 mm pieces and around18pieces are embedded indecided musclewith a marker stitch or clip. If in postoperative period patient still presents with primary HPT features; transplanted area is re-explored and further reduction in parathyroid tissue is done.
Wound is closed with proper haemostasis.
E ects of Surgery
Among neuromuscular symptoms of primary HPT, proximal muscle weakness responds better than respiratory muscle weakness by parathyroidectomy.
Among psychiatric illnesses, depression and spatial learning and processing improve well by surgery.
Chapter 15 Surgery for Thyroid and Parathyroid |
465 |
 omplications
omplications
Bone mineral density in hip and lumbar spine becomes better. Nephrocalcinosis is improved by surgery; but hypertension and
renal excretion will not improve much.
Half life of PTH is 4 minutes. On table serial PTH assays are done at several intervals – before dissection of the gland, during dissection and after dissection. 50% reduction in PTH level from baseline in post removal sample is a 96% predictor of complete removal.
Operative failure rate is 1.5 to 6%. Intra-operative PTH assay improves the success rate very much (76% to 94%). Cure rate is de ned as normocalcaemia in 6 months postoperative period.
Radio guided parathyroidectomy using intravenous injection of 20 mCi of 99mTc sestamibi 2 hours before surgery is not routinely practiced but may be useful for removal of adenoma appropriately. Hand held quantitative gamma counter is used intraoperatively on the neck over all parathyroid tissues.
•Primary HPT is commonly sporadic than familial
•Histological difference between adenoma and hyperplasia is difficult to assess
•Gross on table look is preferred method
•Adenomais usually single. Multiple adenomas occur inelderly
•Hyperplasia involves allfour parathyroids
•Parathyroid cyst may be developmental/secondary to degeneration of nodule or adenoma. Cyst aspiration shows clear fluid with high PTH levelin the contentfluid
•Preoperative IV methylene blue 5 mg/kg in 500 ml dextrose saline which stains parathyroids is often used to localize glands
•50 mg of one gland is sufficient to maintain function
•Persistent HPT is one in which HPT persists immediately even after initial surgery,
•Recurrent HPT (initially after first surgery HPT is correctedbut recurs 6 - 12months after surgery)is due to parathyromatosis,development of newadenoma,andhyperplasiaof transplanted parathyroid.
Parathyromatosis is due torupture and spillageof parathyroid tissuein neck,mediastinum and forming functioning nodules there
•Re-exploration and removal in persistent/recurrent HPT needs on table PTH assay. On table PTH level drop more than 50% of preoperative value indicates the success of surgicalremoval
•CT, MRI, PET scan and selective angiography are usually done only for recurrent diseases

466 |
SRB’s Surgical Operations |
Problems in parathyroidectomy
•Permanent hypoparathyroidism
•Recurrent hyperparathyroidism-hypercalcaemia 12months after parathyroidectomy
•Recurrent laryngeal nerve injury
•Often needs additionalthyroidectomy
•Variations in positions ofthe gland especially lower—may be in mediastinum
•Sudden drop in calcium level after surgery due to increased absorption of calcium by bones—hungrybone syndrome.
Approaches
Classic Approach (Traditional Approach)
It is under general anaesthesia exploring bilateral neck to remove parathyroid tissue which is con rmed by frozen section biopsy. It shows 95% cure rate with 2% complication rate.
Minimally Invasive Parathyroidectomy (MIP)
It is done in case of single adenoma of parathyroid under regional cervical block anaesthesia usually in ambulatory set up. It is actually unilateral parathyroidectomy with removal of adenoma and involved entire that particular one parathyroid gland using limited neck exploration (small incision of 2-4 cm). Procedure is not useful in multiple adenomas or hyperplasia. Preoperative localization with sestamibi combined with SPECT is a must. Intraoperative PTH assay is a must to con rm drop in PTH level to required level. Cervical block is given by in ltrating the xylocaine (1%) with adrenaline along posterior and deep to sternocleidomastoid muscle and also along the neck incision. Direct eld block along the anterior border of the sternocleidomastoid and over the incision is also used. Postoperative serum calcium and PTH level should be assessed until 7th day.
Median Sternotomy (3%) Extension
Median sternotomy is often needed when parathyroid is in anterior mediastinum along with thymus. Often parathyroids may be 5, 6 or 7 in numbers instead of four.
Video-assisted Parathyroidectomy
(Paolo Miccoli)
It is done in localized single adenoma using multiple ports on one side with intermittent CO2 insu ation and suction irrigation. 1.5 cm incision 1 cm above the sternal notch helps in tactile assessment and dissection.Strap muscles are retracted laterallyand thyroid is retracted medially. Conversion rate is 11%; RLN palsy is 2%.
Endoscopic Parathyroidectomy
Entire parathyroidectomy is done using laparoscopy. It is rst used (1994) for a mediastinal parathyroid adenoma. In 1996, it was used for neck parathyroid hyperplasia by Gagner for removing 3½ glands. Now technique is limited to single adenoma to remove tumour and gland. Low pressure insu ations with 5 mm four trocars are used. Placement
of trocars is dependent on the need of the operating surgeon. Many place trocars on one side only; but few prefer to place working trocars on opposite side.
Remedial Parathyroidectomy
It is done for persistent HPT (serum calcium is not normalized immediately after surgery leading total failure) or recurrent HPT (serum calcium after surgery becomes normal but in 6-12 months, it again increases). It is done through lateral approach (Feind) between anterior margin of sternocleidomastoid and strap muscles so that scar tissues are avoided from initial dissection. On table localisation methods, on table PTH assay are needed. Median sternotomy often may be needed to explore the mediastinum for complete removal of the gland. Parathyroids in such situations may be embedded in the tissue of thyroid or thymus. So removal of these glands also may be a need in such situations.
Subtotal Parathyroidectomy
It is indicated in hyperplasia or secondary HPT wherein 3½ glands are removed, retaining ½ of one gland.
Total Parathyroidectomy with Parathyroid Autotransplantation
It is done alternatively in hyperplasia wherein all four glands are removed;⅓or½ ofoneglandistransplantedintosternocleidomastoid or brachioradialis muscle with a marker. Disease control will not occur if more part of the gland is transplanted. It is removing all four glands and 1/2 or 1/3 of one gland is autotransplanted into the forearm muscle (brachioradialis) or sternocleidomastoid muscle with marker stitch. Transplanting gland is sliced into 1 mm pieces and around 18 pieces are embedded in decided muscle with a marker stitch or clip. If postoperatively still patient presents with primary HPT features; transplanted area is re-explored and further reduction in parathyroid tissue is done. Migration or inability to identify the transplanted parathyroid is often a problem in autotransplantation of parathyroid.
 PARATHYROID CARCINOMA
PARATHYROID CARCINOMA
It is rare but shows marked hypercalcaemia (more than 14 mg/dl). Renal and bone complications are more common. Palpable neck swelling,featuresofhypercalcaemia, high PTH level are typical. Lymph nodes in neck may be involved. Tumour is large grayish white, invasive to adjacent thyroid. Distant spread occurs to lung, liver and bone. En bloc parathyroid resection, ipsilateral thyroid lobe removal, adjacent soft tissue resection along with ipsilateral neck lymph node dissection if needed – is the treatment. Simple parathyroidectomy should not be done as it shows high recurrence rate (50%) and spread. Recurrence rate for en bloc removal is 8%. Nodal and distant spread carries poor prognosis. Cinacalcet (30-180 mg/day) is used for symptomatic control of hypercalcaemia. Removal of metastatic area may be needed to control severe hypercalcaemia. Chemotherapy is not useful. External radiotherapy may be used to neck and metastatic area; but results are not adequate.

Chapter 15 Surgery for Thyroid and Parathyroid |
467 |
Figs 15-46(1)

468 |
SRB’s Surgical Operations |
Figs 15-46(2)
Chapter 15 Surgery for Thyroid and Parathyroid |
469 |
Figs 15-46(3)
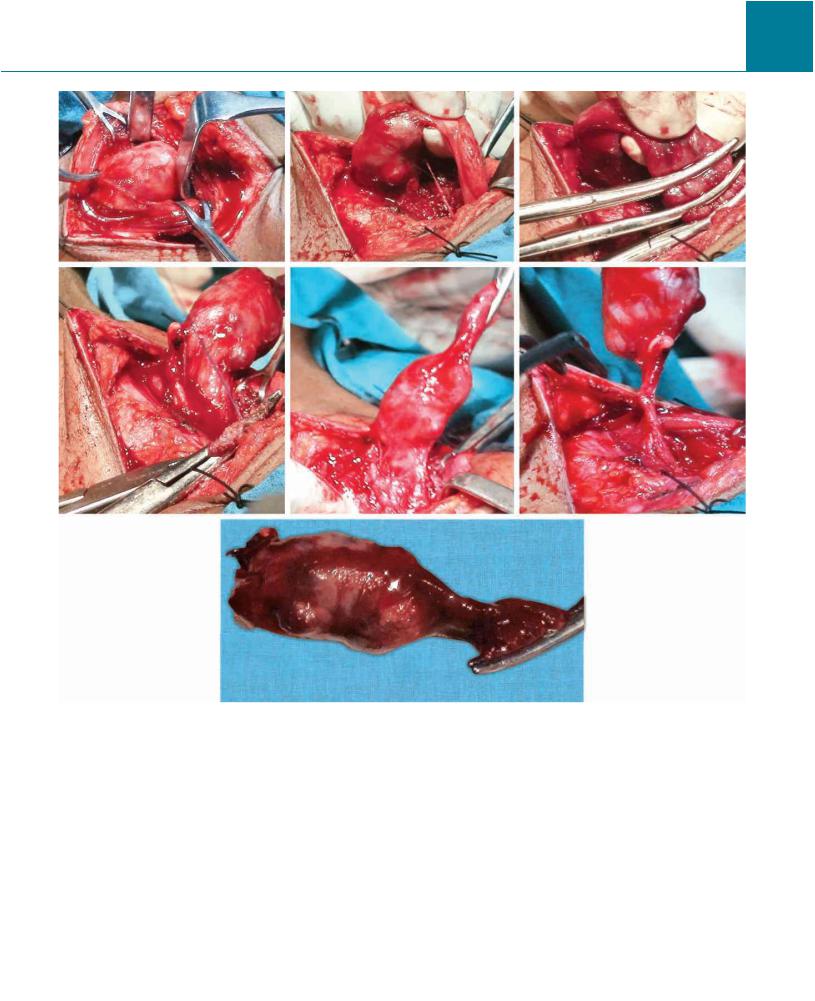
Figs 15-46: Technique of parathyroidectomy for adenoma in two different patients.

Chapter |
|
16 |
Surgeries for |
|
Breast Diseases |
 SURGICAL ANATOMY OF BREAST
SURGICAL ANATOMY OF BREAST
Breast, a modi ed sweat gland lies in super cial fascia in pectoral region with a small extension called as axillary tail of Spence into the axilla through foramen of Langer in deep fascia. It extends from 2nd to 6th rib; from lateral border of sternum to midaxillary line. Deep to breast, structures related are – retromammary space containing loose areolar tissue, pectoral deep fascia, muscles (pectoralis major, serratus anterior, external oblique), chest wall (Fig. 16-1).
Retromammary bursa/space is located between deep layer of super - cial fascia and pectoral (deep) fascia allowing free mobility of breast.
Nipple is located at the level of 4th intercostal space just below the centre/summit of the breast. It contains circular and longitudinal muscles to make nipple sti or at. It is pierced by 15 – 20 lactiferous ducts. Each duct independently opens into the nipple. It has rich sensory nerve endings. It also contains modi ed sweat and sebaceous glands. Nipple is supplied by 4th intercostal nerve.
Areola is circular pigmented area around the nipple. It is rich in modied sebaceous glands which enlarge during pregnancy and lactation
as Montgomery tubercles. ey secrete oily lubricant to nipple and areola. Areola and nipple do not contain hair and fat beneath.
Breast parenchyma contains 15-20 lobes. Each lobe contains alveoli, lactiferous sinus and lactiferous duct. Alveolus is lined by cuboidal (in rest) and columnar (in lactation); smaller duct is by single layer columnar; larger ducts by many layered columnar; lactiferous duct (2-4 mm in diameter) is by strati ed squamous epithelium. Myoepithelial cells lie between epithelium and basement membrane from alveoli to duct. Stroma contains brous tissue and fat. Fibrous stromal septa, anchoring from skin to pectoral fascia, is called as suspensory ligament of Cooper. Fat is distributed all over the breast except under nipple and areola (Fig. 16-2).
Arterial Supply
It is by - Internal thoracic mammary artery (60%), branch of subclavian artery through its perforators; lateral thoracic (30%), superior thoracic and acromiothoracic branches of axillary artery; lateral branches of posterior intercostal arteries. oracodorsal branch of subscapular artery is not supplying the breast but its location and pathway is
Fig. 16-1: Axillary tail of Spence, located deep to deep fascia, is an extension of main breast from super cial fascia through foramen of Langer.

Fig. 16-2: Cooper’s ligament.
important because it is very close to central and scapular (posterior) axillary lymph nodes. So during lymph node dissection injury of this vessel can cause signi cant damage; and it is essential to retain this branch. If LD ap is needed at a later period (Fig. 16-3).
Fig. 16-3: Arterial supply of breast.
Venous return is by - Posterior intercostals with perforating branches which is also in continuity with Batson’s vertebral plexus; axillary vein (primary); internal mammary vein. Venous network form an anastomotic venous circle near base of the nipple which eventually sets into super cial and deep veins. Super cial veins drain into internal thoracic vein; deep into internal thoracic, axillary and posterior intercostal veins.
Nerve supply of breast is from anterior and lateral cutaneous branches of the 4th to 6th intercostal nerves. ey supply skin, smooth muscles, and blood vessels. ey do not control milk secretion. Nipple is supplied by 4th intercostal nerve.
Milk secretion is controlled by prolactin.
Lymphatic drainage of breast – Super cial lymphatics drain skin over the breast except nipple and areola. Deep lymphatics drain parenchyma, nipple and areola. 75% drain into axillary nodes; 20%
Chapter 16 Surgeries for Breast Diseases |
471 |
into internal mammary nodes (parasternal – along internal thoracic vessels deep to the costal cartilage); 5% to posterior intercostal nodes. In axilla, lymphatics mainly drain to pectoral nodes (anterior/external mammary along the lateral thoracic vessels); then to posterior (along subscapular vessels), lateral (along axillary vein), central (in the fat in centre of the axilla) and apical (subclavicular/Halsted) nodes (along pectoralis minor tendon). Supraclavicular, deltopectoral (cephalic), posterior intercostal nodes, subdiaphragmatic lymph plexus are other drainage areas. Sappey’s subareolar lymphatic plexus drains into pectoral group of lymph nodes. Central group is the most properly clinically palpable axillary node. Internal mammary nodes are located in retrosternal intercostal spaces 1-2 cm lateral to the sternal margin; it is vertically placed parallel to internal mammary vessels in relation to endothoracic fascia; its e erent ends in subclavicular nodes.
Intercostal lymph nodes are located posteriorly in intercostal spaces near the origin of ribs. Its e erent drains into the right lymphatic duct or thoracic duct.
Diaphragmatic nodes are located on the thoracic surface of the diaphragm. Anterior prepericardial nodes are located under xiphoid process;lateral one is located oneach side, right side nearIVC, left side near oesophagealhiatus;posterior groupis located nearcrura of diaphragm.
Axillary reverse mapping (ARM) – Here blue dye is injected on inner upper part of the arm to achieve a mapping of axillary lymphatic drainage (Fig. 16-4).
Fig. 16-4: Lymphatic drainage of the breast.
Axilla
Axilla is pyramidal in shape with apex, base and four walls – anterior, posterior, medial and lateral. Apex (cervicoaxillary canal) is bound by clavicle, scapula and outer border of rst rib. Anterior wall is formed by pectoralis major, clavipectoral fascia; posterior wall by subscapularis, teres major and latissimus dorsi; medial wall by upper four ribs with intercostal muscles, upper part of serratus anterior muscle; lateral
