
- •Contents
- •Preface
- •Contributors
- •1 Vessels
- •1.1 Aorta, Vena Cava, and Peripheral Vessels
- •Aorta, Arteries
- •Anomalies and Variant Positions
- •Dilatation
- •Stenosis
- •Wall Thickening
- •Intraluminal Mass
- •Perivascular Mass
- •Vena Cava, Veins
- •Anomalies
- •Dilatation
- •Intraluminal Mass
- •Compression, Infiltration
- •1.2 Portal Vein and Its Tributaries
- •Enlarged Lumen Diameter
- •Portal Hypertension
- •Intraluminal Mass
- •Thrombosis
- •Tumor
- •2 Liver
- •Enlarged Liver
- •Small Liver
- •Homogeneous Hypoechoic Texture
- •Homogeneous Hyperechoic Texture
- •Regionally Inhomogeneous Texture
- •Diffuse Inhomogeneous Texture
- •Anechoic Masses
- •Hypoechoic Masses
- •Isoechoic Masses
- •Hyperechoic Masses
- •Echogenic Masses
- •Irregular Masses
- •Differential Diagnosis of Focal Lesions
- •Diagnostic Methods
- •Suspected Diagnosis
- •3 Biliary Tree and Gallbladder
- •3.1 Biliary Tree
- •Thickening of the Bile Duct Wall
- •Localized and Diffuse
- •Bile Duct Rarefaction
- •Localized and Diffuse
- •Bile Duct Dilatation and Intraductal Pressure
- •Intrahepatic
- •Hilar and Prepancreatic
- •Intrapancreatic
- •Papillary
- •Abnormal Intraluminal Bile Duct Findings
- •Foreign Body
- •The Seven Most Important Questions
- •3.2 Gallbladder
- •Changes in Size
- •Large Gallbladder
- •Small/Missing Gallbladder
- •Wall Changes
- •General Hypoechogenicity
- •General Hyperechogenicity
- •General Tumor
- •Focal Tumor
- •Intraluminal Changes
- •Hyperechoic
- •Hypoechoic
- •Nonvisualized Gallbladder
- •Missing Gallbladder
- •Obscured Gallbladder
- •4 Pancreas
- •Diffuse Pancreatic Change
- •Large Pancreas
- •Small Pancreas
- •Hypoechoic Texture
- •Hyperechoic Texture
- •Focal Changes
- •Anechoic Lesion
- •Hypoechoic Lesion
- •Isoechoic Lesion
- •Hyperechoic Lesion
- •Irregular (Complex Structured) Lesion
- •Dilatation of the Pancreatic Duct
- •Marginal/Mild Dilatation
- •Marked Dilatation
- •5 Spleen
- •Nonfocal Changes of the Spleen
- •Diffuse Parenchymal Changes
- •Large Spleen
- •Small Spleen
- •Focal Changes of the Spleen
- •Anechoic Mass
- •Hypoechoic Mass
- •Hyperechoic Mass
- •Splenic Calcification
- •6 Lymph Nodes
- •Peripheral Lymph Nodes
- •Head/Neck
- •Extremities (Axilla, Groin)
- •Abdominal Lymph Nodes
- •Porta Hepatis
- •Splenic Hilum
- •Mesentery (Celiac, Upper and Lower Mesenteric Station)
- •Stomach
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •Small/Large Intestine
- •Focal Wall Changes
- •Extended Wall Changes
- •Dilated Lumen
- •Narrowed Lumen
- •8 Peritoneal Cavity
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Anechoic Structure
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Wall Structures
- •Smooth Margin
- •Irregular Margin
- •Intragastric Processes
- •Intraintestinal Processes
- •9 Kidneys
- •Anomalies, Malformations
- •Aplasia, Hypoplasia
- •Cystic Malformation
- •Anomalies of Number, Position, or Rotation
- •Fusion Anomaly
- •Anomalies of the Renal Calices
- •Vascular Anomaly
- •Diffuse Changes
- •Large Kidneys
- •Small Kidneys
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Irregular Structure
- •Circumscribed Changes
- •Anechoic Structure
- •Hypoechoic or Isoechoic Structure
- •Complex Structure
- •Hyperechoic Structure
- •10 Adrenal Glands
- •Enlargement
- •Anechoic Structure
- •Hypoechoic Structure
- •Complex Echo Structure
- •Hyperechoic Structure
- •11 Urinary Tract
- •Malformations
- •Duplication Anomalies
- •Dilatations and Stenoses
- •Dilated Renal Pelvis and Ureter
- •Anechoic
- •Hypoechoic
- •Hypoechoic
- •Hyperechoic
- •Large Bladder
- •Small Bladder
- •Altered Bladder Shape
- •Intracavitary Mass
- •Hypoechoic
- •Hyperechoic
- •Echogenic
- •Wall Changes
- •Diffuse Wall Thickening
- •Circumscribed Wall Thickening
- •Concavities and Convexities
- •12.1 The Prostate
- •Enlarged Prostate
- •Regular
- •Irregular
- •Small Prostate
- •Regular
- •Echogenic
- •Circumscribed Lesion
- •Anechoic
- •Hypoechoic
- •Echogenic
- •12.2 Seminal Vesicles
- •Diffuse Change
- •Hypoechoic
- •Circumscribed Change
- •Anechoic
- •Echogenic
- •Irregular
- •12.3 Testis, Epididymis
- •Diffuse Change
- •Enlargement
- •Decreased Size
- •Circumscribed Lesion
- •Anechoic or Hypoechoic
- •Irregular/Echogenic
- •Epididymal Lesion
- •Anechoic
- •Hypoechoic
- •Intrascrotal Mass
- •Anechoic or Hypoechoic
- •Echogenic
- •13 Female Genital Tract
- •Masses
- •Abnormalities of Size or Shape
- •Uterus
- •Abnormalities of Size or Shape
- •Myometrial Changes
- •Intracavitary Changes
- •Endometrial Changes
- •Fallopian Tubes
- •Hypoechoic Mass
- •Anechoic Cystic Mass
- •Solid Echogenic or Nonhomogeneous Mass
- •14 Thyroid Gland
- •Diffuse Changes
- •Enlarged Thyroid Gland
- •Small Thyroid Gland
- •Hypoechoic Structure
- •Hyperechoic Structure
- •Circumscribed Changes
- •Anechoic
- •Hypoechoic
- •Isoechoic
- •Hyperechoic
- •Irregular
- •Differential Diagnosis of Hyperthyroidism
- •Types of Autonomy
- •15 Pleura and Chest Wall
- •Chest Wall
- •Masses
- •Parietal Pleura
- •Nodular Masses
- •Diffuse Pleural Thickening
- •Pleural Effusion
- •Anechoic Effusion
- •Echogenic Effusion
- •Complex Effusion
- •16 Lung
- •Masses
- •Anechoic Masses
- •Hypoechoic Masses
- •Complex Masses
- •Index
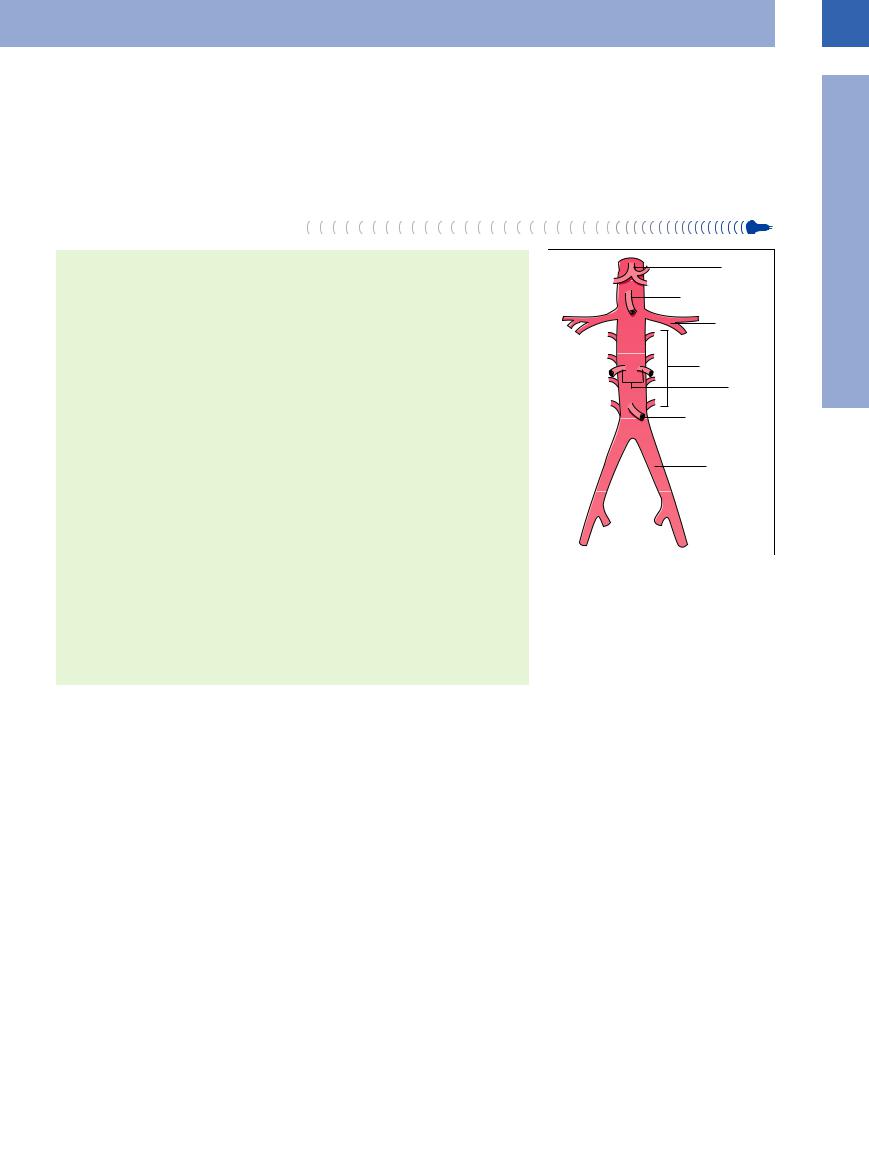
1Vessels
1.1Aorta, Vena Cava, and Peripheral Vessels
G. Schmidt
Aorta: Anatomy and Topography
and Topography
Microanatomy
● Intima (endothelial cells), media (smooth-muscle cells, elastic fibers), adventitia (fibrocellular connective tissue, vascular nerves)
● Thickness of intima media usually 0.4–0.7 mm
Retroperitoneal Branches of the Abdominal Aorta
● Lumbar arteries
● Left and right common iliac arteries
Splanchnic Branches of the Abdominal Aorta
● Superior suprarenal arteries ● Celiac axis
● Left gastric artery ● Splenic artery
● Common hepatic artery → gastroduodenal artery, right gastric artery, hepatic artery proper
● Middle suprarenal arteries |
|
|
|
|
|
|
● Superior mesenteric artery |
|
|
|
|
|
|
● Ovarian/testicular arteries |
|
|
|
|
|
|
● Inferior mesenteric artery |
|
|
|
|
|
|
|
|
|
|
|
||
Course of the Aorta |
|
|
|
|
|
|
● Left of midline and approximately 5 mm anterior to the spine |
Fig. 1.1 Abdominal aorta with its branches. |
|||||
● It enters the abdomen through the aortic hiatus at the level of the lower border of the |
||||||
|
|
|
|
|
||
12th thoracic vertebra and runs for about 14 cm to the level of the fourth lumbar |
|
|
|
|
|
|
vertebra where it bifurcates into the common iliac arteries. In turn these bifurcate into |
|
|
|
|
|
|
the internal and external iliac arteries |
|
|
|
|
|
|
Diameter of the Aortic Lumen
●Normal diameter of the aortic lumen immediately below the diaphragm is 25 mm, gradually tapering down to 20 mm at the infrarenal location
Microanatomy. The intima, the innermost layer of the aorta, is made up of the luminal endothelium and a matrix of fibrils and fibers, while the media as the thickest layer is organized as concentric lamellae of elastin and collagen meshworks with embedded smoothmuscle cells. In the more peripheral arteries the media is characterized by a particular abundance of smooth-muscle cells. The adventitia of the aorta is composed of fibrocellular connective tissue and contains a network of vasa vasorum and vascular nerves. The arterial wall, and thus the aortic wall as well, is a key element in the context of atherosclerosis and its clinical manifestation of arteriosclerosis since the latter results in intimal and medial thickening that can be measured by ultrasonography (intima-media thickness3).1,2 These pathological changes are due to lipid accumulation, proliferation of smooth-muscle cells, and fibroblastic connective tissue.
Ultrasonography depicts the arterial and aortic wall as a triple-layered structure with a hypoechoic layer sandwiched between two hyperechoic strata. The degree of atherosclerosis correlates quite well with the intima-media
thickness, which can be measured using highresolution scanners with high-frequency probes (7.5–10 MHz). The changes in wall thickness are a function of both age and atherosclerosis; for example, the normal wall of the carotid artery is 0.4–0.7 mm thick (see Fig.1.2). The sonographic measurements match the thickness determined histologically.
Retroperitoneal branches of the abdominal aorta. The abdominal aorta gives off paired retroperitoneal as well as (mostly intraperitoneal) splanchnic branches. The retroperitoneal branches include the lumbar arteries, which are of no significance in ultrasonography, and the common iliac arteries, which are essential guideline structures and important in ultrasound pathology (Fig.1.1).
Splanchnic branches of the abdominal aorta.
With the advent of color-flow Doppler scans, imaging of the splanchnic branches of the aorta has played an increasingly important clinical role in organ studies and the detection of disease. Acute chronic intestinal ischemia is easily diagnosed using color-flow Doppler (see
Fig.1.29, p.18). All splanchnic branches (Fig.1.1) are quite accessible to ultrasonography.
Course of the abdominal aorta. The abdominal aorta runs over a distance of about 14 cm from the aortic hiatus of the diaphragm at the level of the lower border of the 12th vertebra to the level of the 4th lumbar vertebra where it bifurcates into the common iliac arteries. At its beginning it passes posterior to the esophagus, which turns left toward the esophageal hiatus and is easily distinguishable by its target sign anterior to the aorta (Fig.1.3). It then runs as a smooth straight band not more than 5 mm anterior to the spine, hugging its curvature; thus it follows the lumbar lordosis in an increasingly anterior direction.
Diameter of the aortic lumen. The maximum diameter of the upper intra-abdominal aortic lumen is 25 mm, tapering down to 20 mm at the infrarenal region; any larger diameter signifies ectasia (< 30 mm) or an aneurysm (> 30 mm) (see Figs. 1.14, 1.15, 1.16, 1.1,
1.1, 1.2).
1.2).
1
Vessels
3
1
Vessels
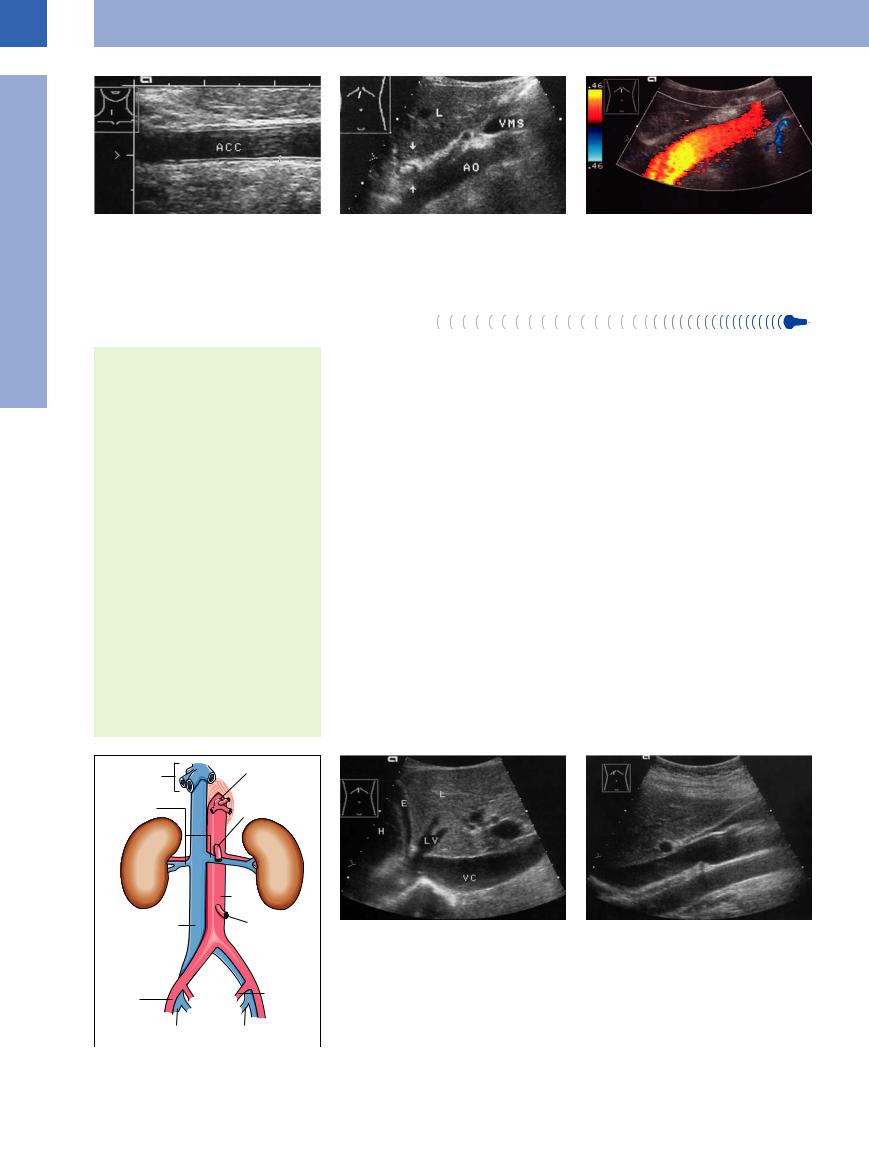
Fig. 1.2 Intima-media thickness of the right common carotid artery (ACC): regular arterial wall with triple layering as a result of reflection from the interfaces between intima, media, and adventitia. In this case the intimamedia thickness is measured as 0.5 mm (cf. the calipers).
Fig. 1.3 Abdominal aorta—course. Longitudinal section. a The upper section of the abdominal aorta runs posterior to the liver. The esophagus junction (arrows) is shown in transverse section; VMS = vena mesenterica superior, L = liver, AO = aorta).
b In color-flow Doppler, laminar flow toward the probe is coded red.
Inferior Vena Cava: Anatomy and Topography
and Topography
Retroperitoneal and Splanchnic Branches
(excluding the splenic vein, superior and inferior mesenteric vein, left and right gastric vein = tributaries to the portal vein)
●Left and right common iliac vein
●Lumbar veins
●Left and right renal vein
●Right ovarian/testicular veins (the corresponding left veins drain into the left renal vein)
●Hepatic veins
Course of the Inferior Vena Cava
●Right of the midline, anterior to the spine, and paralleling the aorta
Lumen Diameter of the Inferior Vena
Cava
●Physiologic caliber changes
●Lumen fluctuates with respiration
●A diameter in excess of 20 mm is pathological: more precisely, it is pathological if the inferior vena cava shows a loss of kinetics—i. e., no change in caliber with respiration or on compression
Microanatomy. The venous wall is quite thin, consisting of smooth-muscle cells and fibrocellular collagen bundles.
The low intrinsic pressure and the thin wall combine to make the inferior vena cava susceptible to caliber changes and extrinsic compression. Therefore, adjacent pathological processes may result in compression, impression, and displacement (see Figs.1.76, 1.78, 1.79, 1.80).
Retroperitoneal and splanchnic branches. All retroperitoneal branches mirror the arterial tree in that they are tributaries to the vena cava, but only some of the splanchnic veins (renal, suprarenal, ovarian/testicular, and hepatic veins) drain into the inferior vena cava (Fig.1.4) and the others into the portal vein (splenic, mesenteric, gastric, and pancreatic veins).
Course of the vena cava. The right and left common iliac veins join at the level of the 5th lumbar vertebra and thus give rise to the inferior vena cava. It courses right of the aorta and anterior to the spine, hugging the lumbar lordosis. It passes along the posterior surface of the liver dorsal to the caudate lobe, receives the vein of the caudate lobe and, at the “bare area,” the three hepatic veins as well. It then enters the thoracic cavity through the central tendon of the diaphragm, immediately terminating in the right atrium (Fig.1.5).
Lumen diameter of the inferior vena cava.
Since the inferior vena cava demonstrates physiological as well as respiration-induced caliber changes it does not have a constant and precise lumen diameter. Nevertheless, a subphrenic diameter of more than 20 mm may be regarded as abnormal when associated with a lack of respiration-induced changes in the lumen diameter and an absence of compressibility.
Fig. 1.5 Inferior vena cava and its branches.
a Upper section of the subphrenic inferior vena cava (VC): it courses posterior to the liver bordering the caudate lobe, just before it is joined by the hepatic veins (LV). H = right atrium; E = small pleural effusion; L = liver.
Fig. 1.4 Inferior vena cava and its branches.
b Longitudinal plane through the inferior vena cava with probe angled from right to left: In this image the vena cava is uppermost, being crossed posteriorly by the right renal vein. Since the aorta runs to the left of the vena cava in this image, it appears at the bottom (this atypical plane provides the best view of any lymphomas between aorta and vena cava). The cross-section of the portal vein with an oblique view through the gallbladder is anterior to the vena cava.
4
