
книги студ / Color Atlas of Pharmacology
.pdf
|
|
|
Disinfectants |
291 |
Application sites |
Examples |
|
Active principles |
|
|
Disinfection of floors |
1. Oxidants |
|
|
|
e. g., hydrogen peroxide, |
|
||
|
or excrement |
|
||
|
potassium permanganate, |
|
||
|
|
|
|
|
|
|
|
peroxycarbonic acids |
|
Inanimate material: durable |
Phenols |
NaOCl |
|
|
|
|
|
|
|
against chemical + physical |
|
Cationic |
|
|
measures |
|
|
|
|
|
|
surfactants |
|
|
|
Disinfection |
2. Halogens |
|
|
|
of instruments |
|
|
|
|
Cationic surfactants |
chlorine |
|
|
Inanimate matter: |
Aldehydes |
sodium hypochlorite |
|
|
iodine tincture |
|
|||
sensitive to heat, |
|
|
|
|
acids, oxidation etc. |
|
|
3. Alcohols |
|
|
Skin disinfection |
|
|
|
|
Regular |
e.g., hands |
R-OH (R=C2-C6) |
|
|
Alcohols |
Phenols |
e. g., ethanol |
|
|
isopropanol |
|
||
Skin |
Cationic surfactants |
4. Aldehydes |
|
|
|
|
|||
|
Acute, |
|
e. g., formaldehyde |
|
|
e.g., before local procedures |
glutaraldehyde |
|
|
|
Iodine |
Chlor- |
5. Organic acids |
|
|
|
|
||
|
tincture |
hexidine |
|
|
|
|
|
e. g., lactic acid |
|
|
Disinfection |
6. Phenols |
|
|
|
of mucous membranes |
|
||
|
|
|
||
|
Chlor- |
|
|
|
Mucous membranes |
hexidine |
|
|
|
|
|
|
|
|
|
Wound disinfection |
|
|
|
|
Chlor- |
KMnO4 |
Nonhalogenated: |
|
|
hexidine |
e. g., phenylphenol |
|
|
|
|
|
eugenol |
|
|
H2O2 |
thymol |
|
|
|
halogenated: |
|
||
|
|
|
chlormethylphenol |
|
Tissue |
|
|
7. Cationic |
|
|
|
STOP |
surfactants |
|
|
|
Cationic soaps |
|
|
|
Disinfectants do |
e. g., benzalkonium |
|
|
|
not afford selective |
chlorhexidine |
|
|
|
inhibition of |
8. Heavy metal salts |
|
|
|
bacteria |
|
||
|
viruses, or fungi |
|
|
|
|
|
|
e. g., phenylmercury borate |
|
A. Disinfectants
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.


|
Antiparasitic Agents |
293 |
|
Tapeworms |
|
Louse |
|
e.g., beef |
|
|
|
tapeworm |
|
|
|
Spasm, |
|
|
|
injury of |
|
|
|
integument |
|
|
|
|
|
Chlor- |
|
|
|
phenothane |
|
|
death |
(DDT) |
|
Praziquantel |
|
|
|
convulsions, |
|
|
|
Round- |
|
|
|
worms, |
|
|
|
e.g., |
system: |
|
|
ascaris |
|
|
|
|
|
|
|
Pinworm |
nervous |
Flea |
|
Damage to |
|
||
|
|
|
|
|
|
Hexachlorocyclo- |
|
Mebendazole |
|
hexane (Lindane) |
|
|
|
|
|
Trichinella |
|
|
|
larvae |
|
Scabies mite |
|
A. Endoand ectoparasites: therapeutic agents
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

|
|
|
Antiparasitic Drugs |
295 |
|||
|
|
|
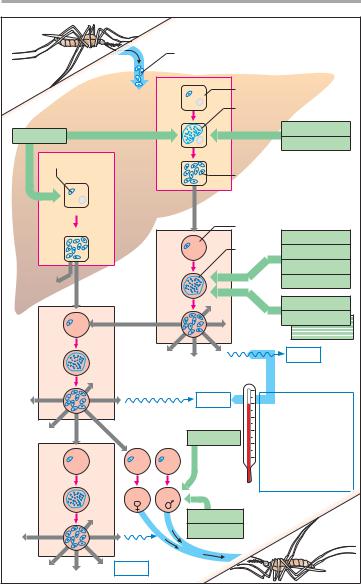
Sporozoites |
|
|
|
|
|
|
|
Hepatocyte |
|
|||
|
|
|
Primary tissue schizont |
|
|||
Primaquine |
Preerythrocytic |
|
|
Pl. falcip. |
Proguanil |
|
|
|
|
Pyrimethamine |
|||||
|
1-4 weeks |
|
|
|
|||
Hypnozoite |
Merozoites |
|
|
||||
|
|
|
|||||
|
|
|
|
|
|
||
|
Only |
|
|
|
|
|
|
|
Pl. vivax |
|
|
|
|
|
|
|
Pl. ovale |
|
Erythrocyte |
|
|
||
|
|
|
|
|
|||
|
|
|
Blood |
|
Chloroquine |
|
|
|
cycle |
|
schizont |
Mefloquine |
|
||
|
|
|
|
|
|
||
|
|
|
|
|
Halofantrine |
|
|
|
Erythrocytic |
|
|
|
|
|
|
|
|
|
|
|
Quinine |
|
|
|
|
|
|
|
Proguanil |
|
|
|
|
|
|
|
Pyrimethamine |
||
|
|
|
|
|
|
||
|
|
|
|
|
|
Sulfadoxine |
|
|
|
|
|
|
|
Fever |
|
|
|
|
Fever |
|
2 days : |
|
|
|
|
|
|
|
Tertian malaria |
|
|
|
|
|
|
|
Pl. vivax, Pl. ovale |
||
|
|
|
|
|
3 days: |
|
|
|
|
|
Primaquine |
|
Quartan malaria |
|
|
|
|
|
|
|
Pl. malariae |
|
|
|
|
|
|
|
No fever |
|
|
|
|
|
|
|
periodicity: |
|
|
|
|
|
|
|
Pernicious malaria: |
||
|
|
|
|
|
Pl. falciparum |
|
|
|
Gametocytes |
|
|
|
|
|
|
|
Fever |
|
|
|
|
|
|
A. Malaria: stages of the plasmodial life cycle in the human; |
|
|
|||||
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.


|
|
Anticancer Drugs |
297 |
Malignant tissue |
Cytostatics inhibit |
Healthy tissue |
|
with numerous mitoses |
cell division |
with few mitoses |
|
Wanted effect: |
|
Little effect |
|
inhibition of |
|
|
|
tumor growth |
|
|
|
|
Healthy tissue with |
Lymph node |
|
|
numerous mitoses |
|
|
|
|
Inhibition of |
|
|
|
lymphocyte |
|
|
|
multiplication: |
|
Damage to hair follicle |
|
immune |
|
Hair loss |
|
weakness |
|
|
|
Lowered resistance to |
|
|
|
infection |
|
|
Unwanted |
Bone marrow |
|
|
|
|
|
|
effects |
Inhibition of |
|
|
|
granulo-, |
|
Inhibition of |
|
thrombocyto-, |
|
|
and erythropoiesis |
|
|
ephithelial renewal |
|
|
|
|
Germinal |
|
|
Diarrhea |
cell damage |
|
|
A. Chemotherapy of tumors: principal and adverse effects
Inhibition of |
Microtubules |
Inhibition of |
of mitotic spindle |
||
formation |
Vinca |
degradation |
|
|
|
|
alkaloids |
Paclitaxel |
Vinca rosea |
|
Western yew tree |
B. Cytostatics: inhibition of mitosis
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.


|
|
|
|
Anticancer Drugs |
299 |
|
DNA |
|
|
|
|
|
|
|
|
Damage |
|
|
|
|
|
|
to template |
|
|
|
|
|
|
Alkylation |
|
|
|
|
|
|
e. g., by |
|
|
|
|
|
|
mechlor- |
|
|
|
|
|
|
ethamine |
|
|
|
|
|
|
Insertion of |
|
|
|
|
|
|
daunorubicin, |
|
|
|
|
|
|
doxorubicin, |
|
|
|
|
|
|
bleomycin, |
|
|
|
|
|
1 |
actinomycin D, etc. |
Streptomyces bacteria |
|
||
|
|
|
|
|||
|
|
|
Inhibition of nucleotide synthesis |
|||
|
|
Building blocks |
|
|
|
|
|
|
Purines |
Tetrahydro- |
Dihydrofolate |
|
|
|
|
|
|
|
||
|
|
Thymine |
folate |
Reductase |
|
|
|
|
|
Folic acid |
|||
|
|
Nucleotide |
|
|
||
RNA |
|
Inhibition by |
|
|
|
|
|
|
|
|
|
||
|
|
Aminopterin |
|
|
|
|
|
|
Methotrexate |
|
|
|
|
|
2 |
|
|
|
|
|
DNA |
DNA |
|
Insertion of incorrect building blocks |
|||
|
|
Purine antimetabolite |
|
|
|
|
|
|
6-Mercaptopurine |
instead of |
Adenine |
|
|
|
|
from Azathioprine |
|
|
|
|
|
|
Pyrimidine antimetabolite |
|
|
|
|
|
|
5-Fluorouracil |
instead of |
Uracil |
|
|
|
|
Cytarabine |
Cytosine |
|
Cytosine |
|
|
3 |
Arabinose |
instead of |
Desoxyribose |
||
|
|
|
|
|
|
|
A.Cytostatics: alkylating agents and cytostatic antibiotics (1), inhibitors of tetrahydrofolate synthesis (2), antimetabolites (3)
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

