
книги студ / Color Atlas of Pharmacology
.pdf
|
|
|
|
|
|
|
|
|
|
|
|
Hormones |
261 |
|||||
|
|
|
|
B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Time |
|
|
|
|
|
|
|
|
|
|
L |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
L |
Healthy |
|
|
|
|
|
|
|
|
|
|
absorption |
|
|
|
|
|
S |
|
subject |
|
Carbohydrate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
B = Breakfast |
|
|
sugar |
|
|
elease |
|
|
|
|
|
|
|
|
|
|
||
S = Snack |
|
|
|
|
|
eas |
|
|
|
|
|
|
|
|
||||
L = Lunch |
|
|
|
|
pancr |
|
|
|
|
|
|
D |
|
|||||
D = Dinner |
|
|
r |
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
N = Supper |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
B |
absorption |
Blood |
Insulin |
|
om |
|
|
Carbohydrate |
|
sugar |
|
|
elease |
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
fr |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
depot |
|
|
||||||
|
L |
|
|
|
|
|
|
|
Blood |
r |
|
|
||||||
|
|
|
|
|
|
|
|
|
Insulin |
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
om |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
fr |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Feast |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
||
|
|
|
|
|
|
|
Glucose |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
L |
|
|
|
|
no |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
lunch |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B |
|
|
|
|
|
|
|
|
|
|
|
Feast |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
L |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B |
|
|
|
|
|
|
Diabetic |
|
D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
L |
|
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A. Control of blood sugar in healthy and diabetic subjects
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.


|
Hormones |
263 |
|
Insulin binding |
|
|
Normal receptor number |
|
Insulin receptor |
Normal |
|
binding |
diet |
|
needed |
|
|
for euglycemia |
Decreased |
|
|
|
|
|
receptor number |
|
|
Obesity |
|
|
Insulin concentration |
|
A. Insulin concentration and binding in normal and overweight subjects
|
|
|
Oral |
|
|
|
anti- |
|
|
|
diabetic |
Insulin release |
|
|
|
Time |
|
|
|
Glucose in blood |
|
|
|
|
|
Diagnosis: |
|
|
|
latent |
overt |
|
|
Diabetes mellitus |
|
Therapy of |
1st |
choice |
Therapy of 2nd choice |
B. Development of maturity-onset diabetes
Membrane |
K+ |
Blockade |
Sulfonylurea derivatives |
depolarization |
|
|
|
|
|
|
|
Insulin |
|
|
|
ATP |
|
Glucose |
Tolbutamide |
B cell |
|
|
C. Action of oral antidiabetic drugs
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

|
|
|
|
|
|
Hormones |
265 |
Electrical |
|
|
|
|
|
|
|
excitability |
|
|
|
|
|
|
|
|
|
|
|
|
|
Bone trabeculae |
|
|
~1 x 10-7M |
|
|
|
|
Hydroxyapatite crystals |
|
|
function |
|
|
|
|
|
|
Ca2+ |
1 x 10-3M |
Ca2+ + PO43- |
Ca10(PO4)6(OH)2 |
|
|||
|
|
||||||
Muscle cell |
Gland cell |
cell |
|
|
|
|
|
on |
|
|
|
|
|
||
|
|
~1 Ca |
|
Osteoclast |
|
||
|
|
Effect |
|
|
|||
|
~10-5M |
x |
|
|
|
||
|
|
|
|
|
|||
|
10 Ca |
|
|
|
|||
|
|
|
- |
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
M |
|
|
|
|
|
|
|
Albumin Globulin |
|
|
|
|
Ca2+ |
|
|
|
|
|
||
Contraction |
Secretion |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Skin |
|
|
|
Parathyroid hormone, Ca2+ |
, PO3- |
|
|
|
|
|
|
|
|
4 |
|
|
25 |
|
25 |
|
|
|
1 |
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
7-Dehydrocholesterol |
|
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
Cholecalciferol |
25-Hydroxychole- |
1,25-Dihydroxychole- |
||||
|
(vitamin D3) |
calciferol |
calciferol (calcitriol) |
||||
|
50-5000 g/day |
(calcifediol) |
0,5-2 g/day |
|
|||
Cod liver oil |
|
|
|
|
|
|
|
|
|
|
|
|
|
Vit. D-Hormone |
|
Parafollicular |
|
Ca2+ |
|
|
Parathyroid |
|
|
cells of |
|
|
|
glands |
|
|
|
thyroid |
|
|
|
|
|
|
|
Calcitonin |
|
|
Parathyroid |
|
|
||
|
|
hormone |
|
|
|||
|
|
|
|
|
|
||
|
|
|
|
|
|
Ca2+ + PO3- |
|
|
|
|
|
|
|
4 |
|
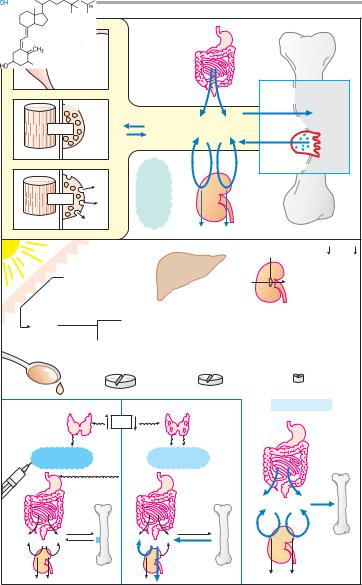
A. Calcium homeostasis of the body
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.


|
|
|
Antibacterial Drugs |
267 |
||
|
|
Anti- |
|
|
|
|
|
|
bacterial |
|
|
|
|
Bacterial |
|
drugs |
|
|
|
|
invasion: |
|
|
|
|
|
|
infection |
|
|
|
|
|
|
|
|
Selective |
|
|
|
|
|
|
antibacterial |
|
|
|
|
|
Immune |
toxicity |
|
|
|
|
|
|
Body cells |
Bacteria |
|
||
1. |
defenses |
|
|
|||
|
|
|
|
|
|
|
|
Penicillins |
Bacitracin |
Polymyxins |
|
|
|
|
Cephalosporins |
Vancomycin |
Tyrothricin |
|
|
|
Cell wall |
|
DNA |
RNA |
|
Cell |
|
|
Tetrahydro- |
|
|
|
membrane |
|
|
|
|
|
|
|
|
|
folate |
|
|
Protein |
|
|
|
synthesis |
|
|
|
|
|
|
Sulfonamides |
Rifampin |
|
Tetracyclines |
|
|
Bacterium |
Trimethoprim |
|
Aminoglycosides |
|
|
|
|
|
|
|
|||
|
|
|
|
Chloramphenicol |
|
|
|
"Gyrase-inhibitors" |
|
Erythromycin |
|
|
|
|
Nitroimidazoles |
|
Clindamycin |
|
|
|
2. |
|
|
|
|
|
|
|
1 day |
|
|
Resistance |
|
|
|
|
|
|
|
|
|
Antibiotic |
|
|
|
|
|
|
|
|
|
Insensitive strain |
|
|
|
|
Bactericidal |
|
|
|
|
|
|
Bacteriostatic |
Sensitive strain with |
Selection |
|
||
3. |
resistant mutant |
|
|
|||
|
|
|
||||
|
|
|
|
|
|
|
A. Principles of antibacterial therapy
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.


|
|
|
Antibacterial Drugs |
|
269 |
||||
Cell wall |
|
|
|
|
|
|
|
|
|
Cell membrane |
|
|
|
|
|
|
|
|
|
Bacterium |
|
|
|
|
Cross-linked |
|
|||
|
|
|
|
|
by |
|
|
|
|
|
|
|
|
|
transpeptidase |
||||
Inhibition of |
|
|
Amino acid |
|
|
|
|
|
|
|
|
chain |
|
|
|
|
|
|
|
cell wall synthesis |
|
|
Cell wall |
|
|
|
|
||
|
Sugar |
|
|
|
|
|
|||
|
|
|
|
building block |
|
||||
|
|
|
Human |
|
Antibody |
|
|||
|
|
|
|
|
|
||||
|
|
|
Penicillin |
|
|
|
|
|
|
|
|
|
allergy |
|
|
|
|
|
|
|
|
|
Neurotoxicity |
|
|
|
|
|
|
|
|
|
at very |
|
|
|
|
|
|
Fungus |
|
|
high dosage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Penicillium notatum |
|
|
|
|
|
|
|
|
|
Plasma concentration |
|
|
Penicillin |
|
|
+ |
|
|
|
|
|
|
|
ocaine |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pr |
|
|
|
|
3 x Dose |
|
|
|
|
Penicillin |
|
|
|
|
|
|
|
~1 |
|
|
|
|
|
|
Minimal |
|
|
|
|
|
+ |
|
||
|
|
|
|
Clemizole |
|
||||
bactericidal |
|
|
|
|
|
- |
|
||
|
|
|
|
|
|
|
|||
concentration |
|
|
(d) |
|
|
|
|
||
|
|
|
|
|
|
|
|||
|
|
Probenecid |
|
Penicillin |
|
|
|||
|
|
action |
|
|
|
|
|
+ |
|
|
|
|
~2 |
|
|
|
+ |
||
|
|
|
|
|
|
- |
|||
|
|
Anion |
BenzathinePenicillins |
||||||
|
|
|
|
||||||
|
|
of |
|
|
|||||
|
|
secretory |
|
|
|||||
|
|
Duration |
|
|
|||||
|
|
system |
|
|
|||||
|
|
|
|
2 |
|
|
|
||
Time |
|
|
~7-28 |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
Increasing the dose |
Combination with probenecid |
Depot preparations |
|||||||
A.Penicillin G: structure and origin; mode of action of penicillins; methods for prolonging duration of action
Lüllmann, Color Atlas of Pharmacology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

