
- •Department of Soil Science & Soil Conservation
- •Introduction
- •2. General scheme & processes of soil formation.
- •3. Morphological features of the soil profile.
- •4. Soil ecology.
- •Study outline:
- •1. Soil definition and the factors of plant growth.
- •2. Plant roots and soil relations.
- •3. Soil fertility and soil productivity.
- •4. Soil texture.
- •1. Sources and composition of som.
- •2. Residue decomposition and humus formation.
- •3. Agronomical and ecological roles of som.
- •4. Maintenance and balance of som.
- •2. Nature and properties of soil colloids.
- •3. Pole in soil genesis and soil productivity development.
- •4. Types and practical significance of soil absorbing capacity.
- •2. Soil Properties as Effected by Exchangeable Cations.
- •3. Soil Acidity & Acid Soil Amendment.
- •4.Soil Alkalinity & Sodic Soil Amendment.
- •5. Soil Buffer Capacity & Significance of Soil pH.
- •2. Managing soil structure.
- •3. Particle density and bulk density.
- •4. Soil porosity and aeration porosity.
- •5. Mechanical properties of mineral soils and their management.
- •2. Soil Water Movement.
- •3. Plant and Soil Water Relations.
- •4.Soil Water Regime.
- •6. Soil Water Management.
- •1.1. Composition and concentration of soil solution.
- •1.2. Osmotic pressure of soil solution.
- •1.3. Redox potential and redox processes in the soils.
- •2. Soil air, a gaseous phase of the soil.
- •2.1. Soil air composition and properties.
- •2.2. Plant requirements to soil aeration.
- •3. Management of soil redox and aeration regimes.
- •1. Soil temperature & modes of energy transfer.
- •2. Conduction of heat in soil. Heat-related soil properties.
- •3. Thermal conductivity of soil.
- •4. Thermal regime of soil profiles &its control.
- •2. Principles of soil cover zoning in Ukraine.
- •3. Soil Zoning in the Mountain regions.
- •4. Fao nomenclature of soils.
- •2. Soddy Podzolic and Soddy Podzolic Gleyed soils.
- •3. Soddy soils.
- •4. Bog and Peat soils.
- •5. Practices of soil management in Ukrainian Polissya.
- •2. Grey Forest and Podzolized soils.
- •3.Chernozems of the Steppe Zone.
- •2. Dark chestnut and chestnut soils.
- •3. Salt-affected soils.
- •4. Practices of soil amendment and land use improvement in the arid steppe zone.
1. Sources and composition of som.
The original source of soil organic matter is plant tissue. Under natural conditions plants usually supply large quantities of organic residues. A good portion of plants are commonly removed from cropped soils, but some of the tops and all of the roots are left in the soil, so that within 2 to 6 mt/ha of residues are left annually in any field crop rotation. Over 70% of material for soil organic matter formation are supplied by the plants and about 30% - by microorganisms. Animals are usually considered secondary sources of OM, though they contribute waste products and leave their own bodies. Certain forms of animal life, especially the earth worms play an important role in the translocation of plant residues.
The composition of higher plant tissue is important for the formation of SOM. About 75% or even more of green tissue of higher plants is water. Over 90% of the dry matter is C, O & H. The other elements play a vital role, especially N, S, P, and Ca. The compounds in plant tissue are many and varied: carbohydrates (sugars, starches and hemicelluloses), fats, waxes, tannins, lignins and proteins. Pre relative resistance of various organic groups to decomposition is different. Sugars, starches, simple and crude proteins are readily decomposed. Some crude proteins and hemicelluloses are decomposed much less readily, while cellulose, fats and waxes are very slowly decomposed.
The components of SOM are classified in accordance with more or les acceptable scheme:


D.S. Orlov proposed a bit more detailed classification.
Some fundamental properties of humic substances are in the
following table:
|
Fulvic Acid |
Humic Acid |
Humin |
Molecular wt. |
1.000-5.000 |
10.000-100.000 |
› 100.000 |
% C |
42-47 |
51-62 |
› 62 |
% O |
45-50 |
31-36 |
‹ 30 |
% N |
2.0-4.1 |
3.6-5.5 |
› 5 |
Acid content (moles/kg) |
14 |
5 |
‹ 5 |
Acid content (mmoles/100g) |
1400 |
500 |
‹ 500 |
CEC, according to D.S.Orlov, mmoles (+)/100g |
300-400 |
600-700 |
-//- |
2. Residue decomposition and humus formation.
The decomposition of SOM is distinctly different from that of original plant material added to the soil. In an experiment, wheat straw was added to soil and the changes in the major components in the straw were followed over time. It was found that proteins, the soluble fraction and the cellulose and hemicellulose disappeared or decomposed very rapidly, where as lignin decomposed very slowly. There was corresponding and rapid increase in microbial products.
Microbial products include living and dead microbial cells and their waste or excretion products. Some of the organic compounds that are synthesized in the soil during decomposition react with each other and with mineral soil components. The decomposition of plant residues result in (1) the production of considerable mass of microbial products, of a considerable mass of mineral products, and (2) the production of a wide variety of materials of varying resistance to decomposition. Labile and stabile fractions of organic matter are produced. These fractions correspond, in general, to the organic residues and humus fractions, respectively. In Ukraine the general scheme of soil humus formation had been proposed by I.V. Tyurin (scheme1).
The labile fraction of SOM consists of any readily degradable materials from plant and animal residues and readily degradable microbial products. The stable SOM consists of resistant compounds: (1) in the decomposing residues, (2) in microbial products and (3) that formed as a
Scheme 1. The general scheme of soil humus formation had been proposed by I.V.Tyurin.
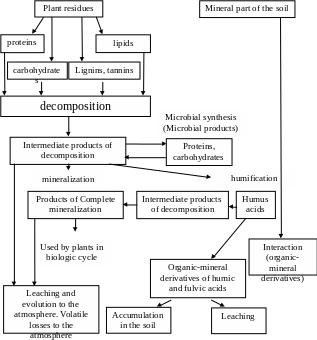
result of interaction of organic compounds with each other and with mineral components of soil, especially the clay.
The stable OM is equivalent to humus. Stable soil organic matter (humus) is heterogeneous mixture of amorphous compounds that are resistant to microbial decomposition and possess a large surface area per gram (up to 1000 m2). This enables humus to absorb water equal to many

COO -

Scheme 2. “Random coil” model of humic substances in soil (Mc Bride. Environmental Chemistry of Soils).
times is weight. C:N ratio is of 10 to 12 to one. Humus is a good source of biologically available N and a significant source of S and P. The CEC of organic matter acts similarly to that of clay particles. Each % of SOM contributes to the soil 30 mmoles (+) of CEC per kilo of oven dry soil. The negative charges arise from exposed – COOH and -OH groups (scheme2). The composition and structure of soil humus is complex and incompletely known. The dissociation of carboxyl and phenol groups yields perhaps 85 to 90% of the negative charge of humus. Many carboxylic groups are sufficiently acidic to dissociate below pH 6. Phenolic OH and very weak acids dissociate at pH›8. The functional groups also buffer soil pH over a wide range.
