
English Lectures / Functional chemistry 1 eng
.pdf
Quantitative determination of magnesium in blood serum
The principle of the method is based on the ability of magnesium with a solution of Magon (1-hydroxyazo-2-naphthol-3-2,4-dimethyl- carboxyanilide) to form a colored complex in an alkaline medium. The color intensity is proportional to the serum magnesium content.

Iron
Iron (6.4-33 mmol/L) is one of the most important trace elements, participating in many vital processes, such as oxygen transport, redox processes in the cell.
•Iron is part of the cytochromes, providing processes of cellular respiration.
•It plays a very important role in the processes of blood formation, the work of the immune system.
•A body normally contains about 4-5 grams of iron, with 70% of it being part of hemoglobin.
•Iron enters the body with food, is found in large quantities in meat, fish (heme iron), and in many vegetables and fruits (non-heme iron).
Iron increase |
Let’s write! |
Excess iron. There is an autosomal recessive hemochromatosis disease associated with excess absorption of iron in the intestine. It is characterized by cirrhosis of the liver, damage to the heart and pancreas, parathyroid glands.
Iron decrease |
Let’s write! |
With iron deficiency in the body, the mobilization of reserves occurs in the following order:
•iron from the depot (ferritin),
•then in the cells (except for erythroid) the number of hemoproteins decreases to a viable minimum,
•further, serum iron reserves (transferrin) are depleted, o hemoglobin synthesis is the last to suffer.
Thus, iron deficiency anemia is a manifestation of extreme iron deficiency.
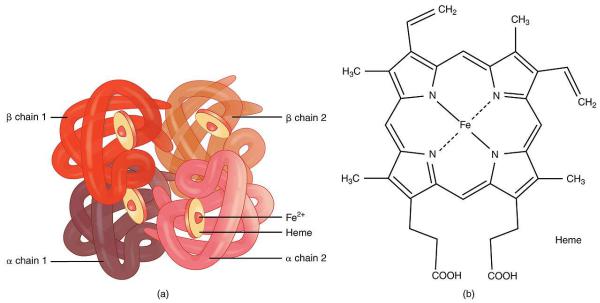
Hemoglobin
Hemoglobin is a complex iron-containing protein of animals with blood circulation, capable of reversibly binding to oxygen, ensuring its transfer to the tissue.
The non-protein part of hemoglobin is heme - a structure that includes a porphyrin ring (consisting of 4 pyrrole rings) and an Fe2 + ion. Iron binds to the porphyrin ring by two coordination and two covalent bonds.
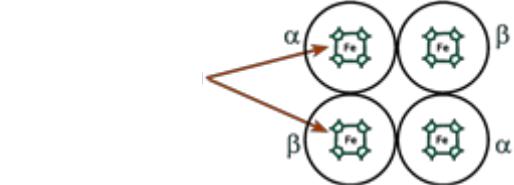
Hemoglobin structure
Hemoglobin is a protein that includes 4 heme-containing protein subunits. The protomers are interconnected by hydrophobic, ionic, hydrogen bonds, while they interact not arbitrarily, but in a specific area - the contact surface. This process is highly specific, contact occurs simultaneously at dozens of points according to the principle of complementarity. The interaction is carried out by oppositely charged groups, hydrophobic sites, and irregularities on the surface of the protein.
heme
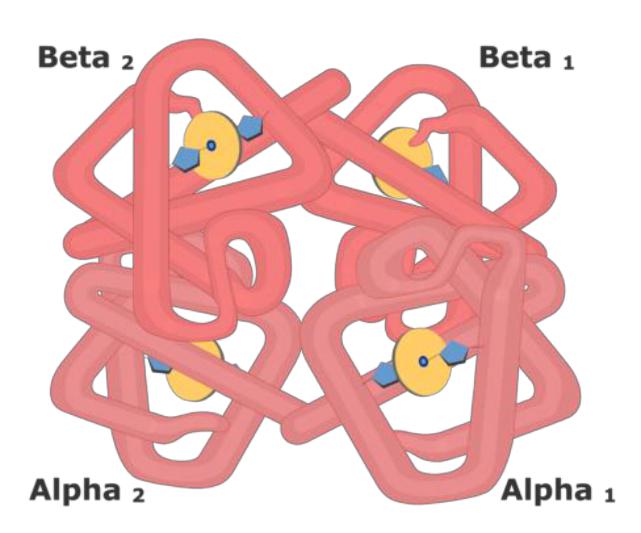
Hemoglobin structure
•The protein subunits in normal hemoglobin can be represented by various types of polypeptide chains: α, β, γ, δ, ε, ξ. A hemoglobin molecule contains two chains of two different types.
•The heme binds to the protein subunit, firstly, through the histidine residue by the coordination bond of iron, and secondly, through hydrophobic bonds of pyrrole rings and hydrophobic amino acids. The heme is located as if "in the pocket" of its chain and a heme-containing protomer is formed.
Let’s write!
Normal forms of hemoglobin
•HbP (primitive) - primitive hemoglobin, contains 2ξand 2ε-chains, occurs in the embryo between 7-12 weeks of life,
•HbF (fetal) - fetal hemoglobin, contains 2α and 2γ chains, appears after 12 weeks of fetal development and is the main after 3 months,
•HbA (adult) - hemoglobin of adults, the proportion is 98%, contains 2α and 2β chains, appears in the fetus after 3 months of life and by birth is 80% of all hemoglobin,
•HbA2 - adult hemoglobin, the proportion is 2%, contains 2α and 2δ chains,
•HbO2 - oxyhemoglobin, is formed by the binding of oxygen in the lungs, in the pulmonary veins its 94-98% of the total amount of hemoglobin,
•HbCO2 - carbohemoglobin, is formed by the binding of carbon dioxide in tissues, in venous blood it makes up 15-20% of the total amount of hemoglobin.
Hemoglobinopathy
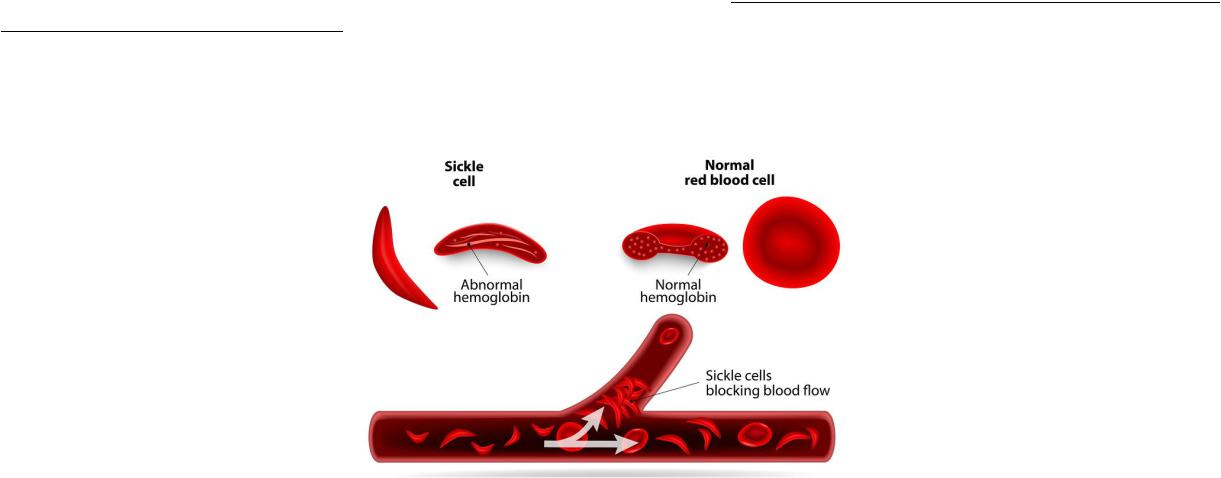
•Hemoglobinopathy – a hereditary or congenital change or violation of the structure of the hemoglobin protein, usually leading to clinically or laboratory-observable changes in its oxygen-transporting function or in the structure and function of red blood cells.
•The most common and known hemoglobinopathies include sickle cell anemia, beta-thalassemia, and persistence of fetal hemoglobin.
•Hemoglobinopathies are classified into qualitative and quantitative. Qualitative ones are due to the replacement of amino acids in polypeptide chains. Replacing the amino acid glutamine 6 with valine in the β- chain leads to the formation of abnormal hemoglobin S, which underlies the development of sickle cell anemia.

Blood oxygen transport
Blood carries out respiratory function primarily due to the presence of hemoglobin in it. The physiological function of hemoglobin as an oxygen carrier is based on the ability to reversibly bind oxygen. Therefore, in the pulmonary capillaries, blood is saturated with oxygen, and in tissue capillaries, where the partial pressure of oxygen is sharply reduced, oxygen is released to the tissues.
