
- •Section I Control of the initial level of knowledge. Biochemical constituents of the cell. Methods of biochemical investigations.
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •77. Discribe the method, shown at the picture below:
- •78. Discribe the method, shown at the picture below:
- •Section іі Enzymes, structure and classification. Regulation of metabolism
- •Е. Whatever part of polypeptide chain of enzyme molecule.
- •Substrate concentration at which reaction rate is half maximal
- •The second enzyme has higher affinity to substrate
- •Competitive
- •Examples of Krok 1 tests
- •Cysteine
- •B. Amylase
- •Peptidases
- •Enteropeptidase
- •Clinical cases and Situational tasks
- •Section ііi Metabolic pathways and bioenergetics. Tricarboxylic acid cycle. Biological oxidation and oxidative phopshorylation
- •1. When atp forms amp:
- •B. Protons
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •Section іv Structure and metabolism of carbohydrates
- •19. Chose the reaction of glycolysis catalyzed by an enzyme phosphofructokinase:
- •A. Liver
- •Examples of Krok 1 tests
- •Acetoacetate, β-hydroxybulyrate, and acetone
- •Clinical cases and Situational tasks
- •Section іv Structure and metabolism of lipids
- •Examples of Krok 1 tests
- •143. A patient with high rate of obesity was advised to use carnitine as a food additive in order to enhance "fat burning". What is the role of carnitine in the process of fat oxidation?
- •144. Lipids are obvious energetic material for the body. What is the main pathway of fatty acids metabolism in mitochondria?
- •Clinical cases and Situational tasks Situational tasks
- •179. The patient is observed an allocation of undigested fat in the faeces. What are the possible causes for this?
- •184. Free cholesterol can affect cholesterol metabolism in the body by inhibiting cholesterol biosynthesis. By which step free cholesterol can inhibit its biosynthesis?
- •186. Explain the mechanism of phospholipids breakdown, shown at the scheme below:
- •Section VI Structure and metabolism of amino acids
- •B. Amylase
- •Examples of Krok 1 tests
- •112. According to clinical indications a patient was administered pyridoxal phosphate. What processes is this medication intended to correct?
- •Clinical cases and Situational tasks
- •145. In a patient 10 g of urine per day is excreted. Evaluate this result.
- •151. Skin color is the aggregate result of the expression of a number of genes modified by ethnic origin and genetic inheritance. What can cause the hypopigmentation?
- •Section VII Principles of molecular biology and molecular genetics
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •108. List and describe properties of the genetic code.
- •113. Fill in the blanks.
- •114. Put the numbers of the enzymes on their place in the picture. Using arrows indicate the direction of replication and direction of synthesis of leading and lagging strands.
- •Section VIII Molecular mechanisms of hormone action on target cells. Biochemistry of hormonal regulation
- •Examples of Krok 1 tests
- •78. For analgesia, a certain substance which imitates the physiological properties of morphine but is synthesized inside the human brain can be used. Name this substance.
- •80. A patient suffering from rheumatism was administered glucocorticoid therapy. What changes in carbohydrate metabolism in liver can be expected?
- •88. In blood of a patient a hypercalcemia, hypophosphatemia, in urine – hyperphosphaturia is observed. What is a possible cause of this state?
- •90. In 13 years old girl a hypotension and polyuria is observed. Preliminary diagnosis – diabetes insipidus. It is caused by deficiency of:
- •93. Signaling via prostanoids begins by interaction of the prostanoid with its receptor. The receptor involved is usually located in which part of the cell?
- •Clinical cases and Situational tasks
- •97. In 13 years old girl a hypotension and polyuria is observed. Preliminary diagnosis – diabetes insipidus. Which hormone deficiency can cause this disease?
- •99. The thyroid hormones t3 and t4 are synthesized in the follicular cells of the thyroid gland. From which of the following essential amino acids are the thyroid hormones synthesized?
- •101. Name types of signalling:
- •Section IX Biochemistry of the nervous tissue
- •С. Ketone bodies
- •24. What compound may be used by the cns cells after extensive physical exercises and prolonged starvation?
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •114. Describe the structure of a synapse and explain how it operates?
- •Section X Biochemistry of the Muscular tissue
- •D. Glycogenolysis in muscles
- •С. Fatigue faster compared to the red fibers
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •Section XI Biochemistry of nutrition
- •1. Note substance, which activates pepsinogen to pepsin:
- •2. Chose the enzyme which plays an important role in production of hydrochloric acid by parietal cells of gastric mucosa glands:
- •3. Which of the following is not a function of the pancreas?
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •62. The clinical and laboratory examination of the patient evaluated the presence of the lactic acid in his gastric juice. What does it indicate? What should be recommended to the patient?
- •69. Discribe the mechanism of hydrochloric acid production shown at the picture:
- •Section XII Functional role of water soluble and fat soluble vitamins in metabolism and providement of cell functions
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •100. A deficiency in thiamine (vitamin b1) would most likely lead to which clinical manifestations?
- •Section XIII Biochemistry and pathobiochemistry of blood
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •89. The blood clotting cascade in humans is represented in the picture below. Using this scheme answer the following questions:
- •Section XIV Functional and clinical biochemistry of liver tissue. Biotransformation of xenobiotics and endogenous toxic compounds
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •Section XV Water and mineral metabolism
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •Section XVI Functional role of kidneys in urinogenesis. Normal and pathological constituents of urine
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •Section XVII Biochemical constituents of connective tissue
- •Examples of Krok 1 Tests
- •Clinical cases and Situational tasks
- •34. Patient with burn disease is at the risk of formation of blood clots in blood vessels. What glycosaminoglycan may be used to prevent formation of blood clots?
- •Section XVIII Biochemistry of saliva and tooth tissue
- •Examples of Krok 1 tests
- •Clinical cases and Situational tasks
- •Section XIX. Biochemical reactions
- •References:
100. A deficiency in thiamine (vitamin b1) would most likely lead to which clinical manifestations?
Answer: In addition to being an important cofactor for the enzymes involved in the oxidative decarboxylation of pyruvate, α-ketoglutarate, and branched-chain α-ketoacids, thiamine is also a cofactor for the enzyme transketolase, the enzyme that transfers a glycoaldehyde group from a ketose sugar to an aldose sugar in the pentose phosphate pathway. One of the diagnostic tools in determining a thiamine deficiency is determination of the activity of red blood cell transketolase in the presence and absence of added thiamine. A thiamine deficiency would be expected to increase blood lactate concentrations. A deficiency of biotin would lead to decreased carboxylase activity, whereas an increased methylmalonate concentration would be observed with a deficiency in vitamin B12. A deficiency in vitamin K would lead to an increase in prothrombin time.
101. Name types of vitamin B6 shown at the picture:
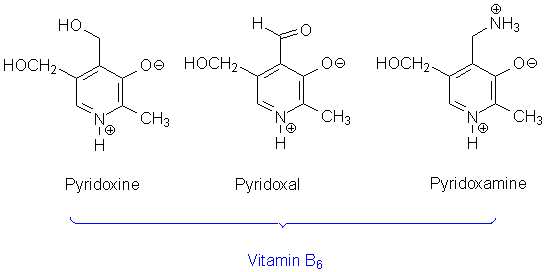
1 2 3
Answer: 1 – pyridoxine, 2 – piridoxal, 3 – piridoxamine.
Section XIII Biochemistry and pathobiochemistry of blood
1. Fibrinogen is transformed into fibrin monomer by the next biochemical process:
Limited proteolytic cleavage
Phosphorylation with involvement of ATP and proteinkinase
Dephosphorylation by protein phosphatase
Carboxylation of glutamic acid residues
Acetylation with involvement of acetyl-CoA.
2. For extrinsic pathway of blood coagulation the next factor is necessary:
Proconvertin (VII)
Hageman factor (XII)
Christmas factor (IX)
Kallicreine
Plasma thromboplastine antecedent (XI)
3. Light chains of immunoglobulins are of following types:
Kappa and lambda
Alpha and kappa
Alpha and gamma
Lambda and delta
Alpha and beta
4. Prothrombin is activated and transformed to thrombin by an active form of the next intrinsic factor:
Plasma thromboplastin antecedent (X)
Convertin (factor VII)
Antihemophilic globulin A (VIII)
Hageman factor (XII)
Christmas factor (IX)
5. Hemophilia A is caused by deficiency of the next blood coagulation factor:
Antihemophilic globulin A
Proconvertin
Proaccelerin
Tissue thromboplastin
Christmas factor
6. Immunoglobulins are classified on the basis of:
Type of heavy chains
Type of light chains
Types of light and heavy chains
Molecular weight
7. Post translational modification of coagulation factors and formation of γ-carboxyglutamic acid has the next functional significance:
Increases the affinity to Ca ions
Decreases the affinity to Ca ions
Induces formation of additional ionic bonds in peptide chain
Induces appearance of Mg ion ligands in protein molecule
Provides cross linking of fibrin filaments
8. The molecular weight of heavy chains of immunoglobulins is:
50,000–70,000 Da
20,000–25,000 Da
25,000–50,000 Da
70,000–1,00,000 Da
1,00,000-5,00,000 Da
9. Thrombin belongs to the next class of enzymes:
Hydrolases
Oxido-reductases
Transferases
Lyases
Isomerases
Ligases
10. Plasminogen is activated and transformed to the active form plasmin by the following enzyme:
Urokinase
Thrombin
Thrombokinase
FSF (fibrin stabilization factor)
Ca ions
11. The most abundant immunoglobulin in plasma is:
IgG
IgA
IgM
IgD
IgE
12. Allergic reactions are mediated by:
IgE
IgA
IgG
IgD
IgM
13. C1 component of classical complement pathway is made up of:
Complements 1q and 1r
Complements 1q and 1s
Complements 1r and 1s
Complements 1q, 1r and 1s
14. Which from listed below immunoglobulins is secretoty and inhibits adsorption and growth of bacteria on mucosa surfaces?
A.IgA
B. IgM
C. IgD
D. IgE
E. IgG
15. IgG cleaved by papain into:
Two Fab and one Fc fragments
Two light and two heavy chains
Two pairs of one light and one heavy chain each
One Fab and two Fc fragments
16. Severe combined immunodeficiency (SCID) is caused by congenital defect of the next enzyme:
Adenosine deaminase
Alanyl aminotransferase
Transketolase
Adenosine phosphorylase
AMP nucleotidase
17. Chose the symbol of heavy chain type in immunoglobulin M:
A. μ
B. β
C. γ
D. λ
E. α
18. Immunoglobulin M molecule possesses the next quaternary structure:
A. It is pentameric molecule
B. It is dimeric molecule
C. It is trimeric molecule
D. It is tetrameric molecule
E. It is monomeric molecule
19. The antibody class which can pass through the placenta to protect the fetus is
A. Immunoglobulin G (IgG)
B. Immunoglobulin M (IgM)
C. Immunoglobulin A (IgA)
D.Immunoglobulin D (IgD)
E.Immunoglobulin E (IgE)
20. The minimum number of polypeptide chains in an immunoglobulin is:
Four
Two
Five
Six
Seven
21. Cross linking of fibrin monomers in the filament and formation of tight clot is provided by the next factor:
FSF (fibrin stabilization factor)
Plasmin
Thromboplastin
Convertin
Accelerin
22. Chose from listed below factors one of the extrinsic pathway of blood coagulation:
Proconvertin
Prothrombine
Antihemophilic globulin A
Hageman factor
Proaccelerin
23. Chose from presented vitamins one with antihemorrhagic activity:
A. Philloquinone
B. Retinol
C. Tocopherol
D. Ergocalciferol
E. Pangamic acid
24. Vitamin K serves as a cofactor in the next enzymatic reaction:
A. Carboxylation of glutamic acid side chain
B. Decarboxylation of glutamic or aspartic acid side chain
C. Limited proteolytic cleavage of zymogens
D. Phosphorylation of serine or threonine
E. Amidation of glutamic acid side chain
25. The molecular weight of light chains of immunoglobulins is:
20,000–25,000
10,000–15,000 Da
25,000–50,000 Da
50,000–75,000 Da
75,000-100,000 Da
26. Secretory component is present in:
IgA
IgG
IgM
IgD
IgE
27. The components of complement system are activated by:
Phosphorylation
Microsomal hydroxylation
Glycosylation
Proteloysis
28. Chose the symbol of light chain type of immunoglobulins:
λ
α
β
γ
μ
29. Hemophilia B is caused by deficiency of the next blood coagulation factor:
A. Christmas factor (factor IX)
B. Proaccelerin
C. Antihemophilic globulin A
D. Tissue thromboplastin
E. Proconvertin
30. Chose the correct value of normal protein concentration in human blood plasma:
A.65-85 g/l
B.45-60 g/l
C.25-40 g/l
D.85-100 g/l
E.100-150 g/l
31. Proteins of blood plasma are divided into albumin and globulins. What is quantitative proportion of albumin to globulins (albumin/globulin coefficient)?
A.1.5:2
B.2:1
C.5:1
D.1:1
E.1:5
32. The three primary types of plasma proteins are:
Albumins, globulins, fibrinogen
Heme, iron, globin
Antibodies, metallo-proteins, lipoproteins
Serum, fibrin, fibrinogen
None of the above
33. The predominant constituent of blood rest nitrogen is:
A. Urea
B. Creatinine
C. Bilirubin
D. Phenylalanine
E. Glutathion
34. One of the components of blood residual nitrogen fraction is bilirubin, which is produced from:
A. Heme
B. Cholesterol
C. Levulinic acid
D. Creatinine
E. Tryptophan
35. Uric acid is a final product of catabolism of the next component of nucleic acids
A. Guanine
B.Phosphate
C.Thymine
D. Ribose
E. Orotat
36. Myoglobin belongs to:
A. Hemoproteins
B. Albumins
C. Glycoproteins
D. Transferrins
E. Phosphoproteins
37. Hemoglobin belongs to?
A. Chromoproteins
B. Nukleoproteins
C. Phosphoproteins
D. Lipoprotein
E. Glycoproteins
38. The structure of heme in hemoglobin is:
A. Protoporphyrin IX, attached to the Fe2+
B. Four pyrrol rings, attached to Fe3+
C. Four pyrrol rings, attached to Fe 2+
D. Porphyrin coupled with Fe
E. Protoporphyrin X, attached to and Fe 3+
39. Protoporphyrin IX belongs to one of the following:
A. Hemoglobins
B. Transferrins
C. Ceruloplasmins
D. Properdins
E. Cryoglobulines
40. Methylene blue promotes oxidation of hemoglobin. Give the name of the obtained compound:
A. Methemoglobin
B. Hematin
C. Hemine
D. Carboxyhemoglobin
E. Carbhemoglobin
41. Thalassemia is a genetic disorder of synthesis of one of the chains of hemoglobin. Inhibition of β - chain synthesis leads to formation of β-thalassemia. Which of the following will be the best feature of the disease?
A. Increased concentration of hemoglobin F
B. Decreased concentration of hemoglobin F
C. Hemolytic anemia.
D. Sickle erythrocyte shape
E. Reduction of hemoglobin A2
42. In complex proteins their prosthetic group is associated with protein moiety. Choose from the list one amino acid responsible for the formation of bonds between these two parts:
A. Histidine
B. Serine
C. Alanine
D. Tyrosine
E. Lysine
43. Choose from the following a major end product of protein metabolism that is excreted in the largest quantity of urine:
A. Ammonia and ammonium salts
B. Glutamine
S. Uric acid
D. Allantoin
E. Urea
44. What from the following belongs to nitrogen-free organic compounds?
A. Vitamin C
B. ATP
C. Glucagon
D. Glutamine
45. What physical and chemical properties of blood explain the presence of electrolytes?
A. Osmotic pressure
B. Oncotic pressure
C. Erythrocyte sedimentation rate
D. Viscosity of blood
46. In erythrocytes carbonic acid is formed from CO2 and H2O. What enzyme provides a synthesis of carbonic acid in erythrocytes and its degradation in the capillaries?
A. Anhydrase
B. Amylase
C. Elastase
D. Alkaline phosphatase
E. Lipase
47. Hemoglobin differs from similar proteins by specific physical and chemical properties. Indicate physical and chemical properties that distinguish hemoglobin from myoglobin?
A. Molecular weight
B. Solubility
C. Electrophoretic activity
D. Spectral properties
48. As a complex protein, hemoglobin consists of protein and non-protein moieties. Indicate the components of hemoglobin.
A. 4 Hem groups, 2 α - and 2 β - polypeptide chains
B. Hem, 1 α - and 3 β - polypeptide chains
C. 4 Hem groups and 4 β - polypeptide chains
D. 4 Hem groups and 4 α -polypeptide chains
E. Hem, 2 α - and 2 β - polypeptide chains
