
- •Surface Preparation and Cleaning. Table of content
- •5.1.1.Hand tool cleaning.
- •6.5.1.2.Power tool cleaning.
- •6.5.2. Blast-cleaning.
- •Figure 6.2.
- •Illustration of various blasting methods
- •6.5.2.1.Centrifugal abrasive blast-cleaning
- •6.5.2.2.Compressed-air abrasive blast-cleaning.
- •Figure 6.3 Effect of nozzle pressure on cleaning rate
- •6.5.2.3.Vacuum or suction-head abrasive blast-cleaning.
- •6.5.2.4.Moisture-injection abrasive blast-cleaning (compressed-air moisture- injection abrasive blast-cleaning)
- •6.5.2.5.Wet abrasive blast-cleaning (Compressed-air wet abrasive blast-cleaning).
- •6.5.2.6. Other methods
- •6.6. Abrasives.
- •Table 6.1. Abrasive / Profile Comparative Chart
- •Table 6.2. How to specify Blasting
- •Table 6.3. Examples of production rates to a Sa 2 ½ condition.
- •Table 6.4
- •Table 6.5. Consumption rates at different Nozzle pressures.
- •6.6.1. Metallic abrasives.
- •Table 6.6.
- •6.7. Water jetting and hydro blasting
- •Figure 6.6 Rotating nozzles must be designed to fit the purpose
- •Figure 6.7 The water used must be free from impurities, which may contaminate the surface.
- •6.8. Electrolytic descaling with magnesium strips
- •Figure 6.8 Principle of electrolytic descaling
- •6.9. Preparation between paint coats
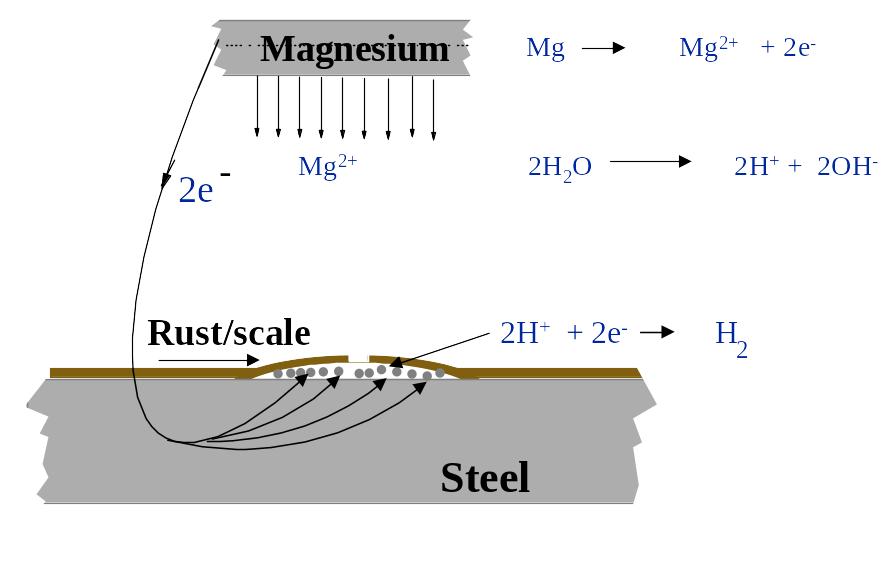
6.8. Electrolytic descaling with magnesium strips
Electrolytic descaling is a very promising alternative for pre-treatment of seawater containing tanks (particularly ballast tanks) as part of a maintenance procedure. It has proven to be very competitive due to the fact that the method removes rust and scale, while the ship is sailing. This method is only relevant for ships in service where extensive corrosion and rusting have occurred in the tank. The method is difficult to use in tanks with a large number of compartments and structures, but this is also the case for the alternatives to descaling. Descaling of deckheads may be poor as a result of air-pockets, and sludge (mud) can reduce the efficiency of the method on the bottom plates.
The principle for electrolytic descaling is based on an extreme form of cathodic protection. Due to its very negative corrosion potential the Magnesium will dissolve (corrode) rapidly. The reaction releases Mg-ions and electrons. The electrons follow the electrical connector from the anode to the steel, creating a very high current output to the steel underneath the rust and scale. At the steel surface the electrons react with hydrogen-ions present in the water and forms hydrogen gas. The Hydrogen will initiate a process of lifting the rust and scale from the steel surface. This happens as dissolution of the magnesium becomes Mg-ions which in turn react with the hydroxide ions in the ballast water to form a calcareous deposits on the steel surface. This process completes the descaling process.
Figure 6.8 Principle of electrolytic descaling
Recommendations for descaling with Magnesium strips
Descaling can be carried out on a voyage in ballast, using lengths of Mg-strips, cut to size. (Maximum 30 metres when connected at both ends). Each anode has a core of iron wires. These are fixed to the hull by means of clamps or by welding. Good electrical contact to the steel is essential. When possible, the distance to the steel should be about 50 cm.
The amount of strips to be installed has to be based on the degree of rusting and the consistency of the scale, as well as the area of the tank. The required amount of Magnesium strips will vary with seawater temperature and salinity. In general, about 0.6 m Mg strips is required per square metre of tank surface. The exact length is determined by inspection.
The strips must be distributed as evenly as possible, if necessary being held in place by lengths of rope. After the strips have been installed, the tank is filled and pressured up with seawater, which should have a temperature of at least 10 oC. Brackish water is insufficiently conductive and thus cannot be used.
Hydrogen is generated under the rust flakes, which therefore loosen without the steel itself being attacked. Since hydrogen generation can result in an explosive atmosphere, very good air circulation is essential. Carefully specified safety measures must be followed, and the conditions in the tank with regard to gas accumulation and oxygen availability must be approved.
The length of time before the tank can be emptied and cleaned is 7 - 14 days, depending on salinity and seawater temperature.
The surface will appear white as a result of the creation of large amounts of calcium carbonate and smaller quantities of magnesium hydroxide. These must be removed by high-pressure water jetting (200 - 400 bar) before they dry, which must be followed up by drying as soon as possible. If the carbonate and hydroxide deposits are not removed, popping, osmotic blisters can be formed, causing subsequent paint coats to flake off.
The main activities involved in a descaling process are:
Empty the ballast tank
Mount the Magnesium strips
Fill the tank and have a ballasting period of approx. 1-2 weeks
Empty the tank and wash down with fresh water at a pressure in the range of 200 to 400 bar
Remove loose rust scale and remaining Mg-strips
Dry the tank with dehumidifying equipment
Apply the first stripe coat and coat of paint
Install sacrificial anodes to back up the coating system
Complete coating work with further stripe coats
It is important to bear in mind that the generation of hydrogen gives rise to an explosion hazard. The ballast tanks must be well ventilated, and the use of open flames or hot equipment must not be permitted near hatches and other openings where a hazardous mixture of hydrogen and oxygen is existing. Furthermore, the tank must be completely filled with seawater during the process to avoid any air pockets where gas can be accumulated.
The method cannot be used for removing rust that contains oil or grease, because the current cannot penetrate to the underlying steel surface. For similar reasons, the method is not effective with magnetite rust.
