
- •Preface
- •List of contributers
- •History, epidemiology, prevention and education
- •A history of burn care
- •“Black sheep in surgical wards”
- •Toxaemia, plasmarrhea, or infection?
- •The Guinea Pig Club
- •Burns and sulfa drugs at Pearl Harbor
- •Burn center concept
- •Shock and resuscitation
- •Wound care and infection
- •Burn surgery
- •Inhalation injury and pulmonary care
- •Nutrition and the “Universal Trauma Model”
- •Rehabilitation
- •Conclusions
- •References
- •Epidemiology and prevention of burns throughout the world
- •Introduction
- •Epidemiology
- •The inequitable distribution of burns
- •Cost by age
- •Cost by mechanism
- •Limitations of data
- •Risk factors
- •Socioeconomic factors
- •Race and ethnicity
- •Age-related factors: children
- •Age-related factors: the elderly
- •Regional factors
- •Gender-related factors
- •Intent
- •Comorbidity
- •Agents
- •Non-electric domestic appliances
- •War, mass casualties, and terrorism
- •Interventions
- •Smoke detectors
- •Residential sprinklers
- •Hot water temperature regulation
- •Lamps and stoves
- •Fireworks legislation
- •Fire-safe cigarettes
- •Children’s sleepwear
- •Acid assaults
- •Burn care systems
- •Role of the World Health Organization
- •Conclusions and recommendations
- •Surveillance
- •Smoke alarms
- •Gender inequality
- •Community surveys
- •Acknowledgements
- •References
- •Prevention of burn injuries
- •Introduction
- •Burns prevalence and relevance
- •Burn injury risk factors
- •WHERE?
- •Burn prevention types
- •Burn prevention: The basics to design a plan
- •Flame burns
- •Prevention of scald burns
- •Conclusions
- •References
- •Burns associated with wars and disasters
- •Introduction
- •Wartime burns
- •Epidemiology of burns sustained during combat operations
- •Fluid resuscitation and initial burn care in theater
- •Evacuation of thermally-injured combat casualties
- •Care of host-nation burn patients
- •Disaster-related burns
- •Epidemiology
- •Treatment of disaster-related burns
- •The American Burn Association (ABA) disaster management plan
- •Summary
- •References
- •Education in burns
- •Introduction
- •Surgical education
- •Background
- •Simulation
- •Education in the internet era
- •Rotations as courses
- •Mentorship
- •Peer mentorship
- •Hierarchical mentorship
- •What is a mentor
- •Implementation
- •Interprofessional education
- •What is interprofessional education
- •Approaches to interprofessional education
- •References
- •European practice guidelines for burn care: Minimum level of burn care provision in Europe
- •Foreword
- •Background
- •Introduction
- •Burn injury and burn care in general
- •Conclusion
- •References
- •Pre-hospital and initial management of burns
- •Introduction
- •Modern care
- •Early management
- •At the accident
- •At a local hospital – stabilization prior to transport to the Burn Center
- •Transportation
- •References
- •Medical documentation of burn injuries
- •Introduction
- •Medical documentation of burn injuries
- •Contents of an up-to-date burns registry
- •Shortcomings in existing documentation systems designs
- •Burn depth
- •Burn depth as a dynamic process
- •Non-clinical methods to classify burn depth
- •Burn extent
- •Basic principles of determining the burn extent
- •Methods to determine burn extent
- •Computer aided three-dimensional documentation systems
- •Methods used by BurnCase 3D
- •Creating a comparable international database
- •Results
- •Conclusion
- •Financing and accomplishment
- •References
- •Pathophysiology of burn injury
- •Introduction
- •Local changes
- •Burn depth
- •Burn size
- •Systemic changes
- •Hypovolemia and rapid edema formation
- •Altered cellular membranes and cellular edema
- •Mediators of burn injury
- •Hemodynamic consequences of acute burns
- •Hypermetabolic response to burn injury
- •Glucose metabolism
- •Myocardial dysfunction
- •Effects on the renal system
- •Effects on the gastrointestinal system
- •Effects on the immune system
- •Summary and conclusion
- •References
- •Anesthesia for patients with acute burn injuries
- •Introduction
- •Preoperative evaluation
- •Monitors
- •Pharmacology
- •Postoperative care
- •References
- •Diagnosis and management of inhalation injury
- •Introduction
- •Effects of inhaled gases
- •Carbon monoxide
- •Cyanide toxicity
- •Upper airway injury
- •Lower airway injury
- •Diagnosis
- •Resuscitation after inhalation injury
- •Other treatment issues
- •Prognosis
- •Conclusions
- •References
- •Respiratory management
- •Airway management
- •(a) Endotracheal intubation
- •(b) Elective tracheostomy
- •Chest escharotomy
- •Conventional mechanical ventilation
- •Introduction
- •Pathophysiological principles
- •Low tidal volume and limited plateau pressure approaches
- •Permissive hypercapnia
- •The open-lung approach
- •PEEP
- •Lung recruitment maneuvers
- •Unconventional mechanical ventilation strategies
- •High-frequency percussive ventilation (HFPV)
- •High-frequency oscillatory ventilation
- •Airway pressure release ventilation (APRV)
- •Ventilator associated pneumonia (VAP)
- •(a) Prevention
- •(b) Treatment
- •References
- •Organ responses and organ support
- •Introduction
- •Burn shock and resuscitation
- •Post-burn hypermetabolism
- •Individual organ systems
- •Central nervous system
- •Peripheral nervous system
- •Pulmonary
- •Cardiovascular
- •Renal
- •Gastrointestinal tract
- •Conclusion
- •References
- •Critical care of thermally injured patient
- •Introduction
- •Oxidative stress control strategies
- •Fluid and cardiovascular management beyond 24 hours
- •Other organ function/dysfunction and support
- •The nervous system
- •Respiratory system and inhalation injury
- •Renal failure and renal replacement therapy
- •Gastro-intestinal system
- •Glucose control
- •Endocrine changes
- •Stress response (Fig. 2)
- •Low T3 syndrome
- •Gonadal depression
- •Thermal regulation
- •Metabolic modulation
- •Propranolol
- •Oxandrolone
- •Recombinant human growth hormone
- •Insulin
- •Electrolyte disorders
- •Sodium
- •Chloride
- •Calcium, phosphate and magnesium
- •Calcium
- •Bone demineralization and osteoporosis
- •Micronutrients and antioxidants
- •Thrombosis prophylaxis
- •Conclusion
- •References
- •Treatment of infection in burns
- •Introduction
- •Clinical management strategies
- •Pathophysiology of the burn wound
- •Burn wound infection
- •Cellulitis
- •Impetigo
- •Catheter related infections
- •Urinary tract infection
- •Tracheobronchitis
- •Pneumonia
- •Sepsis in the burn patient
- •The microbiology of burn wound infection
- •Sources of organisms
- •Gram-positive organisms
- •Gram-negative organisms
- •Infection control
- •Pharmacological considerations in the treatment of burn infections
- •Topical antimicrobial treatment
- •Systemic antimicrobial treatment (Table 3)
- •Gram-positive bacterial infections
- •Enterococcal bacterial infections
- •Gram-negative bacterial infections
- •Treatment of yeast and fungal infections
- •The Polyenes (Amphotericin B)
- •Azole antifungals
- •Echinocandin antifungals
- •Nucleoside analog antifungal (Flucytosine)
- •Conclusion
- •References
- •Acute treatment of severely burned pediatric patients
- •Introduction
- •Initial management of the burned child
- •Fluid resuscitation
- •Sepsis
- •Inhalation injury
- •Burn wound excision
- •Burn wound coverage
- •Metabolic response and nutritional support
- •Modulation of the hormonal and endocrine response
- •Recombinant human growth hormone
- •Insulin-like growth factor
- •Oxandrolone
- •Propranolol
- •Glucose control
- •Insulin
- •Metformin
- •Novel therapeutic options
- •Long-term responses
- •Conclusion
- •References
- •Adult burn management
- •Introduction
- •Epidemiology and aetiology
- •Pathophysiology
- •Assessment of the burn wound
- •Depth of burn
- •Size of the burn
- •Initial management of the burn wound
- •First aid
- •Burn blisters
- •Escharotomy
- •General care of the adult burn patient
- •Biological/Semi biological dressings
- •Topical antimicrobials
- •Biological dressings
- •Other dressings
- •Exposure
- •Deep partial thickness wound
- •Total wound excision
- •Serial wound excision and conservative management
- •Full thickness burns
- •Excision and autografting
- •Topical antimicrobials
- •Large full thickness burns
- •Serial excision
- •Mixed depth burn
- •Donor sites
- •Techniques of wound excision
- •Blood loss
- •Antibiotics
- •Anatomical considerations
- •Skin replacement
- •Autograft
- •Allograft
- •Other skin replacements
- •Cultured skin substitutes
- •Skin graft take
- •Rehabilitation and outcome
- •Future care
- •References
- •Burns in older adults
- •Introduction
- •Burn injury epidemiology
- •Pathophysiologic changes and implications for burn therapy
- •Aging
- •Comorbidities
- •Acute management challenges
- •Fluid resuscitation
- •Burn excision
- •Pain and sedation
- •End of life decisions
- •Summary of key points and recommendations
- •References
- •Acute management of facial burns
- •Introduction
- •Anatomy and pathophysiology
- •Management
- •General approach
- •Airway management
- •Facial burn wound management
- •Initial wound care
- •Topical agents
- •Biological dressings
- •Surgical burn wound excision of the face
- •Wound closure
- •Special areas and adjacent of the face
- •Eyelids
- •Nose and ears
- •Lips
- •Scalp
- •The neck
- •Catastrophic injury
- •Post healing rehabilitation and scar management
- •Outcome and reconstruction
- •Summary
- •References
- •Hand burns
- •Introduction
- •Initial evaluation and history
- •Initial wound management
- •Escharotomy and fasciotomy
- •Surgical management: Early excision and grafting
- •Skin substitutes
- •Amputation
- •Hand therapy
- •Secondary reconstruction
- •References
- •Treatment of burns – established and novel technology
- •Introduction
- •Partial thickness burns
- •Biological membranes – amnion and others
- •Xenograft
- •Full thickness burns
- •Dermal analogs
- •Keratinocyte coverage
- •Facial transplantation
- •Tissue engineering and stem cells
- •Gene therapy and growth factors
- •Conclusion
- •References
- •Wound healing
- •History of wound care
- •Types of wounds
- •Mechanisms of wound healing
- •Hemostasis
- •Proliferation
- •Epithelialization
- •Remodeling
- •Fetal wound healing
- •Stem cells
- •Abnormal wound healing
- •Impaired wound healing
- •Hypertrophic scars and keloids
- •Chronic non-healing wounds
- •Conclusions
- •References
- •Pain management after burn trauma
- •Introduction
- •Pathophysiology of pain after burn injuries
- •Nociceptive pain
- •Neuropathic pain
- •Sympathetically Maintained Pain (SMP)
- •Pain rating and documentation
- •Pain management and analgesics
- •Pharmacokinetics in severe burns
- •Form of administration [21]
- •Non-opioids (Table 1)
- •Paracetamol
- •Metamizole
- •Non-steroidal antirheumatics (NSAID)
- •Selective cyclooxygenasis-2-inhibitors
- •Opioids (Table 2)
- •Weak opioids
- •Strong opioids
- •Other analgesics
- •Ketamine (see also intensive care unit and analgosedation)
- •Anticonvulsants (Gabapentin and Pregabalin)
- •Antidepressants with analgesic effects
- •Regional anesthesia
- •Pain management without analgesics
- •Adequate communication
- •Psychological techniques [65]
- •Transcutaneous electrical nerve stimulation (TENS)
- •Particularities of burn pain
- •Wound pain
- •Breakthrough pain
- •Intervention-induced pain
- •Necrosectomy and skin grafting
- •Dressing change of large burn wounds and removal of clamps in skin grafts
- •Dressing change in smaller burn wounds, baths and physical therapy
- •Postoperative pain
- •Mental aspects
- •Intensive care unit
- •Opioid-induced hyperalgesia and opioid tolerance
- •Hypermetabolism
- •Psychic stress factors
- •Risk of infection
- •Monitoring [92]
- •Sedation monitoring
- •Analgesia monitoring (see Fig. 2)
- •Analgosedation (Table 3)
- •Sedation
- •Analgesia
- •References
- •Nutrition support for the burn patient
- •Background
- •Case presentation
- •Patient selection: Timing and route of nutritional support
- •Determining nutritional demands
- •What is an appropriate initial nutrition plan for this patient?
- •Formulations for nutritional support
- •Monitoring nutrition support
- •Optimal monitoring of nutritional status
- •Problems and complications of nutritional support
- •Conclusion
- •References
- •HBO and burns
- •Historical development
- •Contraindications for the use of HBO
- •Conclusion
- •References
- •Nursing management of the burn-injured person
- •Introduction
- •Incidence
- •Prevention
- •Pathophysiology
- •Severity factors
- •Local damage
- •Fluid and electrolyte shifts
- •Cardiovascular, gastrointestinal and renal system manifestations
- •Types of burn injuries
- •Thermal
- •Chemical
- •Electrical
- •Smoke and inhalation injury
- •Clinical manifestations
- •Subjective symptoms
- •Possible complications
- •Clinical management
- •Non-surgical care
- •Surgical care
- •Coordination of care: Burn nursing’s unique role
- •Nursing interventions: Emergent phase
- •Nursing interventions: Acute phase
- •Nursing interventions: Rehabilitative phase
- •Ongoing care
- •Infection prevention and control
- •Rehabilitation medicine
- •Nutrition
- •Pharmacology
- •Conclusion
- •References
- •Outpatient burn care
- •Introduction
- •Epidemiology
- •Accident causes
- •Care structures
- •Indications for inpatient treatment
- •Patient age
- •Total burned body surface area (TBSA)
- •Depth of the burn
- •Pre-existing conditions
- •Accompanying injuries
- •Special injuries
- •Treatment
- •Initial treatment
- •Pain therapy
- •Local treatment
- •Course of treatment
- •Complications
- •Infections
- •Follow-up care
- •References
- •Non-thermal burns
- •Electrical injury
- •Introduction
- •Pathophysiology
- •Initial assessment and acute care
- •Wound care
- •Diagnosis
- •Low voltage injuries
- •Lightning injuries
- •Complications
- •References
- •Symptoms, diagnosis and treatment of chemical burns
- •Chemical burns
- •Decontamination
- •Affection of different organ systems
- •Respiratory tract
- •Gastrointestinal tract
- •Hematological signs
- •Nephrologic symptoms
- •Skin
- •Nitric acid
- •Sulfuric acid
- •Caustic soda
- •Phenol
- •Summary
- •References
- •Necrotizing and exfoliative diseases of the skin
- •Introduction
- •Necrotizing diseases of the skin
- •Cellulitis
- •Staphylococcal scalded skin syndrome
- •Autoimmune blistering diseases
- •Epidermolysis bullosa acquisita
- •Necrotizing fasciitis
- •Purpura fulminans
- •Exfoliative diseases of the skin
- •Stevens-Johnson syndrome
- •Toxic epidermal necrolysis
- •Conclusion
- •References
- •Frostbite
- •Mechanism
- •Risk factors
- •Causes
- •Diagnosis
- •Treatment
- •Rewarming
- •Surgery
- •Sympathectomy
- •Vasodilators
- •Escharotomy and fasciotomy
- •Prognosis
- •Research
- •References
- •Subject index
Pain management after burn trauma
ally diagnosed cerebrovascular disorders or heart diseases (NYHA II-IV). The risk of an acute renal failure is similar to when administering NSAID.
Selective cyclooxygenasis-2-inhibitors are not approved for persons under 18 years of age.
Parecoxib: Approved for the short-term treatment (48 hours) of post-surgical pain. Recommended dosage is 40 mg and it is administered intravenously or intramuscularly. After a period of 12 hours, another 20 mg or 40 mg can be administered again. There are no special dosage recommendations for older patients. Due to the missing effect on the thrombocyte function, parecoxib is suitable for pain management after skin grafting or debridement.
Celecoxib: Administered orally, daily dosage 200 mg in 1 or 2 single doses.
Opioids (Table 2)
Opioids are the fundamental substances in pain management of severe burn injuries. Due to their various possibilities of application, opioids are suitable for all stages of the treatment.
Apart from the typical side effects as for example depressed breathing, bradycardia, obstipation, nausea and itching, the following facts have to be considered:
After a short time of administration, opioids induce adaptation mechanisms and cause the development of functional antagonisms, which might lead to an opioid tolerance or an opioidinduced hyperalgesia [34, 35]. Whereas in an opioid tolerance the dosage of the analgesic has to be increased to have the same effect, in an opi- oid-induced hyperalgesia the sensation of pain is stronger. Although both phenomena have different pathophysiologic mechanisms, the activation of NMDA-receptors plays a key role in both cases [36–38]. It is recommended to combine opioids with NMDA-receptor antagonists.
Liver and renal disorders cause an alteration of the pharmacokinetics of opioids. In the presence of liver insufficiency an increased bio-availability of morphine, hydromorphone and pethidine through reduction of the first-pass-metabolism has to be anticipated.
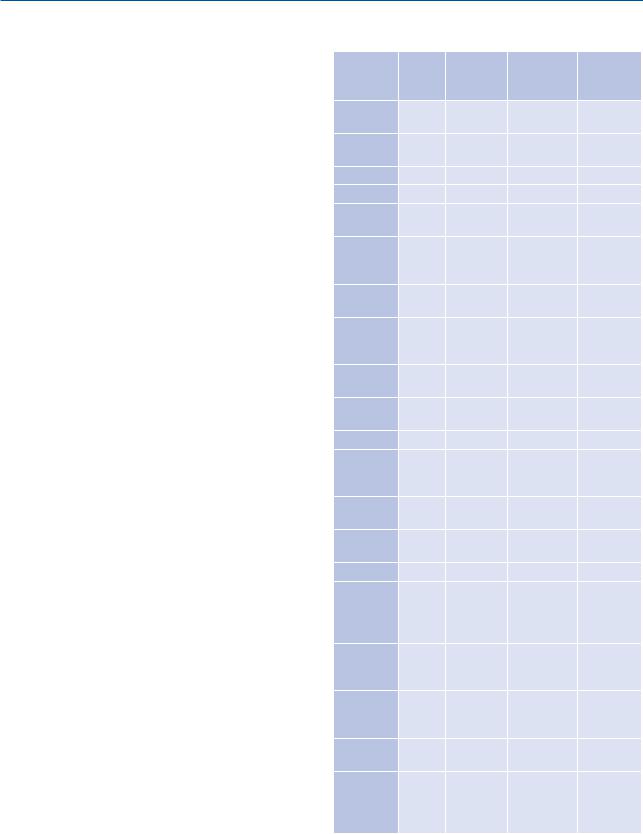
Table 2. Pharmacological data of opioids
Active |
HL (h) |
Adminis- |
Single dose |
Action |
Ingredient |
|
tration |
in adults |
time |
|
|
|
(mg) |
(h) |
Trama- |
6–7 |
p. o. |
from 50 |
4–6 |
dol* |
|
|
|
|
|
|
p. o. |
from 100 |
12 |
|
|
(retard) |
|
|
|
|
i. v. |
100 |
4–6 |
Morphine |
2–3 |
p. o. |
from 10 |
3–4 |
|
|
p. o. |
from 10 |
8–12 |
|
|
(retard) |
|
|
|
|
i. v. |
from 2 |
lock out |
|
|
(PCA- |
|
time |
|
|
dose) |
|
10–15 min |
Fentanyl |
3–4 |
TTS |
from |
72 (48) |
|
|
|
0,6 mg/24h |
|
|
|
i. v. |
30–40 μg |
lock out |
|
|
(PCA- |
|
time |
|
|
dose) |
|
5 min |
Hydro- |
2–3 |
p. o. |
from 1,3 |
4–6 |
morphone |
|
|
|
|
|
|
p. o. |
from 4 |
8–12 |
|
|
(retard) |
|
|
|
|
i. v. |
1–1,5 |
3–4 |
|
|
i. v. |
from 0,2 |
lock out |
|
|
(PCA- |
|
time |
|
|
dose) |
|
5–10 min |
Oxyco- |
4–6 |
p. o. |
from 5 |
4–6 |
done |
|
|
|
|
|
|
p. o. |
from 10 |
8–12 |
|
|
(retard) |
|
|
|
|
i. v. |
1–10 |
4 |
|
|
v. |
0.03 mg/kg |
lock out |
|
|
(PCA- |
|
time at |
|
|
dose) |
|
least |
|
|
|
|
5 min |
Levo- |
20–55 |
p. o. |
from 2 |
6–12 |
metha- |
|
|
|
|
done |
|
|
|
|
|
|
i. v. |
from 0,5 |
lock out |
|
|
(PCA- |
|
time |
|
|
dose) |
|
5–10 min |
Piritram- |
2–4 |
i. v. |
15 |
6–8 |
ide |
|
|
|
|
|
|
|
|
lock out |
|
|
i. v. |
|
time |
|
|
(PCA- |
|
10–15 |
|
|
dose) |
1–2 |
min |
* Max. Daily dosage: 400 mg–600 mg
345

R. Girtler, B. Gustorff
rA renal insufficiency can cause the onset of active metabolites, e. g.:
rMorphine-6-glukuronid: little analgesic but significant sedating effect
rMorphine-3-glukuronid: pronociceptive, excitatoric effect [39]. A cumulation can be coresponsible for the development of an opioid tolerance [34].
rNorpethidine: neurotoxic effect
Thus the administration of opioids should always be embedded in a multimodal treatment. Due to different metabolites and different response rates to the opioid receptors, the opioid rotation is an important option. To administer the correct quantity during a change of opioids it is recommended to start with 50% of the calculated equivalence dosage of the new opioid to be able to titrate as needed. This procedure is reasonable since the present equivalence tables are suited only for opioid naïve patients and due to a tolerance development the dosage equivalence varies greatly.
The genetic polymorphism with multiple isoforms for the μ-, -, and -receptors is the reason why the effects of opioids can interindividually be very different. Thus the dosage must be determined individually. In older or weak patients the initial dosage should be reduced and the effect of the initial dosage should be taken into consideration when further dosages are determined.
Any opioid-caused effect can be reversed immediately and entirely by administering a specific antagonist as for example Naloxon.
are weaker. However, there is a higher incidence of vomiting and nausea. Due to genetic polymorphisms in the cytochrome oxydase system the analgesic effect of Tramadol is limited in some patients (appr. 10% in Caucasians) [40].
Tramadol is also effective in the treatment of neuropathic pain [41]. Neuropathic pain might play a role in chronic pain after burn trauma (see III. Pathophysiology of pain after burn injuries/3. Neuropathic pain). However, the current literature does not provide any evaluation of the effects of Tramadol on neuropathic pain in burn patients.
Pethidine
Although pethidine is slowly but steadily replaced by more modern preparations in numerous European countries, it still remains one of the most important analgesic medication throughout the world. Pethidine is a μ-receptor agonist with a 0.1–0.2 fold analgesic potence of morphine. Onset of action takes place after 5 minutes and lasts for 2 to 3 hours. During degradation the active neurotoxic metabolite norpethidine is produced which, when acculmulated, can cause convulsion and myoclonus. Thus pethidine is suitable for intravenous administration in acute cases but not as continuous treatment. Pethidine reduces the post operative shivering better than morphine. In addition it shows the least spasmogenicity of all opioids. Currently it is particularly used in the initial treatment of children with burn trauma.
Weak opioids
The daily dosage of weak opioids must be obeyed.
Tramadol
Tramadol is an analgesic with a combination of opi- oid-antagonistic effects and re-uptake inhibition of serotonine and noradrenaline. It is available either as delayed-action preparation or non-delayed action preparation. In addition it is available as parenterally injectable pharmacon. It is suitable for the therapy of acute and chronic pain. Compared to morphine and other opioids, Tramadol’s breathing depressing effect and its inhibition of the gastro-intestinal tract
Strong opioids
There is no clinically relevant maximum dosage for strong opioids.
Morphine
In pain management morphine is commonly used as reference opioid throughout the world. It can be administered orally or parenterally. Due to its high first-pass effect its bioavailability is only 20%–40%. When administered orally, the dosage needs to be 3-fold higher than when administered parenterally. Morphine is degraded in the liver to morphine-3- glucuronide (pronociceptive effect) and morphine-
346

Pain management after burn trauma
6-glucuronide (sedating effect). These metabolites show a significantly higher elimination half-life (up to 72 hours) than morphine. In the presence of renal insufficiency both metabolites might accumulate. After intravenous administration the onset of maximum action takes place after 15 to 30 minutes. The action time of a single dosage is 3 to 5 hours. Possible side effects are a dosage-dependent respiratory depression and vasodilatoric effects as well as release of histamines with vasodilatation and bronchoconstriction.
Piritramide
Piritramide is a μ-receptor agonist with a relative activity of 0.7 compared to morphine. It is very well suitable in post-operative intravenous pain management, in the intensive care unit and as patient-con- trolled analgesic. The onset of action takes place after 5 to 10 minutes and generally lasts for 4 to 6 hours. The sedative components are stronger than those of morphine. Piritramide acts hardly euphorizing and its spasmogenicity is low.
Fentanyl
The potency of fentanyl is 100-fold higher than the potency of morphine (analgesic and side effects). A dosage of 100 μg equals approximately 10 mg morphine. It maintains the cardiac stability and has a high therapeutic index. Compared to morphine the histamine release remains low.
Forms of administration:
Intravenous: The onset of maximum action takes place after 4 to 5 minutes and the time of action is 20 to 30 minutes. Fentanyl is officially approved for intraoperative analgesia (with artificial respiration) and for analgosedation in the intensive care unit. However, fentanyl is more and more used in awake patients not receiving artificial respiration: a successful administration of fentanyl in combination with propofol for the analgosedation of non-intubated children (5 to 60 months, ASA II-III, TBSA 5%-25%) during dressing change after skin grafting has been described [42]. Linneman et al. have investigated the effect of fentanyl in 55 burn patients (9 months to 75 years) in a
retrospective study. 31% of the patients developed a transient respiratory depression. However, additional oxygen or intubation have not been necessary for any patient. With adequate monitoring and in the presence of an anesthetist the administration of fentanyl during dressing change is described as effective and secure [43].
Intravenous PCA: The patient-controlled analgesia with fentanyl during dressing change in burn patients is very effective. Signs of circulatory insufficiency or respiratory depression have not been described [44]. Recommended pump programming: bolus of 30 μg fentanyl with a lock-out time of 5 minutes after an initial loading dose of 1 μg/kg fentanyl.
Transdermal: Only suitable for chronic pain management and in the presence of adequate resorption areas. Onset of action takes place after 12 to 24 hours.
Oromucosal: Suitable in case of breakthrough pain despite of a permanent opioid therapy (see also breakthrough pain).
Hydromorphone
Hydromorphone is a μ-receptor agonist and its analgesic potency is 6 to 7.5-fold higher than the analgesic potency of morphine. It can be administered orally (non-retarded, retarded) and parenterally injectably. Hydromorphone shows a favorable pharmacological profile due to its lack of active metabolites and its low plasma protein binding (appr. 8%). Thus it is particularly suitable, also for permanent treatment, for patients suffering from renal insufficiency.
Oxycodone
Oxycodone acts at the μ-receptors and also at the - and -receptors. In case of decreasing analgesic effects of a permanent opioid treatment, the rotation to oxycodone may have positive effects. Apart from that, oxycodone has been described as particularly effective in neuropathic pain [45, 46].
It has a bioavailability of 70% to 87% and its analgesic potency is comparable to morphine. Metabolites of oxycodone are noroxycodone and oxymorphone, both showing only very low analgesic activity. The level of active metabolites in the blood is not sig-
347
