
Книги фарма 2 / Bertram G. Katzung-Basic & Clinical Pharmacology(9th Edition)
.pdf
While the amine hypothesis is undoubtedly too simplistic, it has provided the major experimental models for the discovery of new antidepressant drugs. As a result, all the currently available antidepressant drugs—except bupropion—are classified as having their primary actions on the metabolism, reuptake, or selective receptor antagonism of serotonin, norepinephrine, or both.
Katzung PHARMACOLOGY, 9e > Section V. Drugs That Act in the Central Nervous System > Chapter 30. Antidepressant Agents >
Basic Pharmacology of Antidepressants
Chemistry
A variety of different chemical structures have been found to have antidepressant activity. With the possible exception of bupropion, however, the core antidepressant action of even the newest agents derives from mechanisms engaged by antidepressants introduced 4 decades ago.
Tricyclic Antidepressants (TCAs)
Tricyclic antidepressants—so called because of the characteristic three-ring nucleus—have been used clinically for four decades (Figure 30–1). They closely resemble the phenothiazines chemically and, to a lesser extent, pharmacologically. Like the latter drugs, they were first thought to be useful as antihistamines with sedative properties and later as antipsychotics. The discovery of their antidepressant properties was a fortuitous clinical observation. Imipramine and amitriptyline are the prototypical drugs of the class as mixed norepinephrine and serotonin uptake inhibitors though they also have several other properties.
Figure 30–1.

Structural relationships between various tricyclic antidepressants (TCAs).
Heterocyclics; Secondand Third-Generation Drugs
Between 1980 and 1996, a number of heterocyclic agents denoted as second-generation and thirdgeneration or heterocyclic antidepressants were introduced. Four of the agents classified as "secondgeneration" are available for clinical use in the USA and are shown in Figure 30–2. Amoxapine and maprotiline resemble the structure of the tricyclic agents, while trazodone and bupropion are distinctive. The heterocyclic agents are not notably different from the older agents in potency. Since 1990, venlafaxine, a chemically unique third-generation agent; mirtazapine, an analog of a widely used European antidepressant; and nefazodone, developed on the basis of trazodone, have been introduced. The structures of these compounds are shown in Figure 30–3.
Figure 30–2.
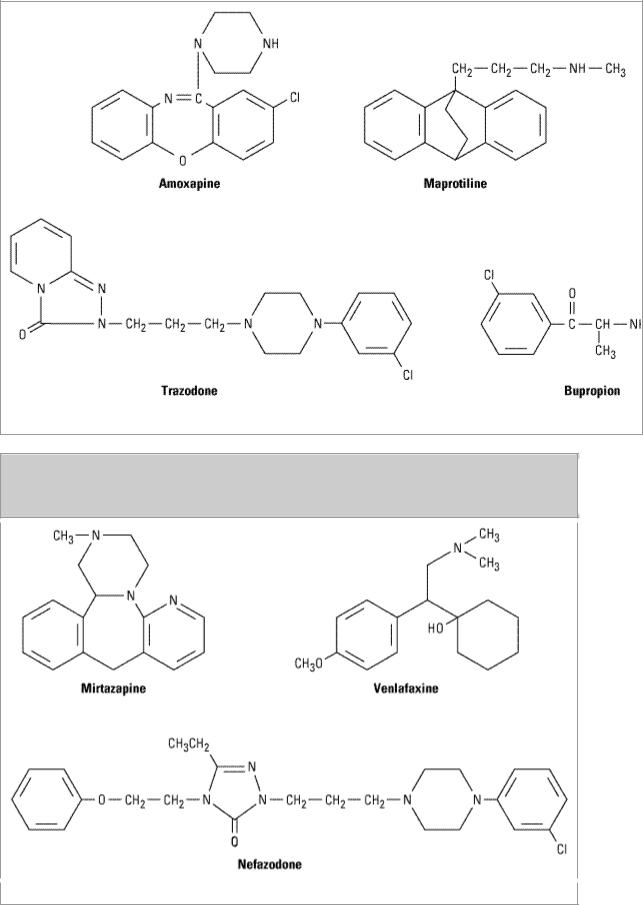
Among the major drawbacks of most first-generation antidepressants have been their many "irrelevant" pharmacologic actions, a trait inherited from the phenothiazine antipsychotic agents. As far as has been determined, the antimuscarinic, antihistaminic, and 
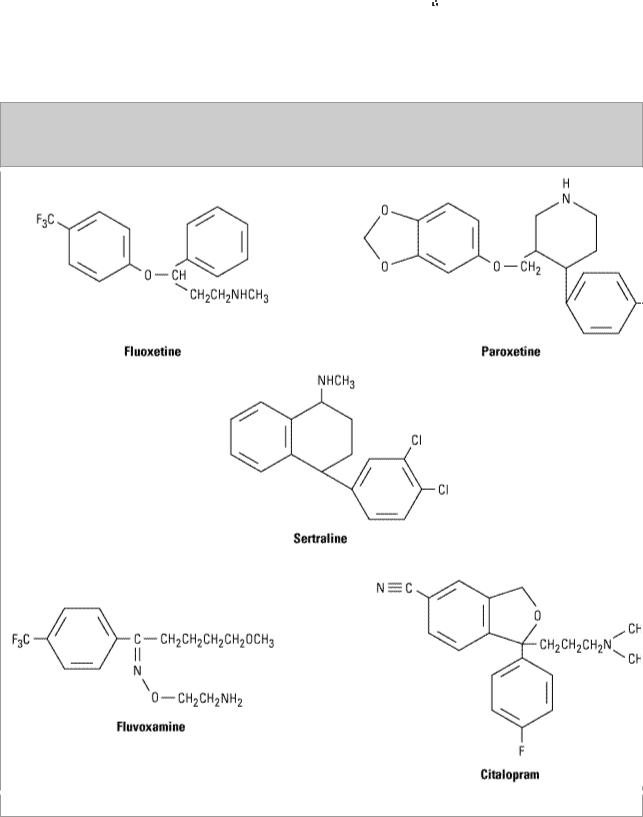
 adrenoceptor-blocking actions of tricyclic antidepressants contribute only to the toxicity of these agents. Since the introduction of fluoxetine—an effective and more selective antidepressant with minimal autonomic toxicity—four more selective serotonin reuptake inhibitors have been introduced as well as the active enantiomeric form of one, (S)-citalopram. All are structurally distinct from the tricyclic molecules (Figure 30–4).
adrenoceptor-blocking actions of tricyclic antidepressants contribute only to the toxicity of these agents. Since the introduction of fluoxetine—an effective and more selective antidepressant with minimal autonomic toxicity—four more selective serotonin reuptake inhibitors have been introduced as well as the active enantiomeric form of one, (S)-citalopram. All are structurally distinct from the tricyclic molecules (Figure 30–4).
Figure 30–4.
Selective serotonin reuptake inhibitors (SSRIs).
Monoamine Oxidase (MAO) Inhibitors
MAO inhibitors may be classified as hydrazides, exemplified by the C–N–N moiety, as is the case
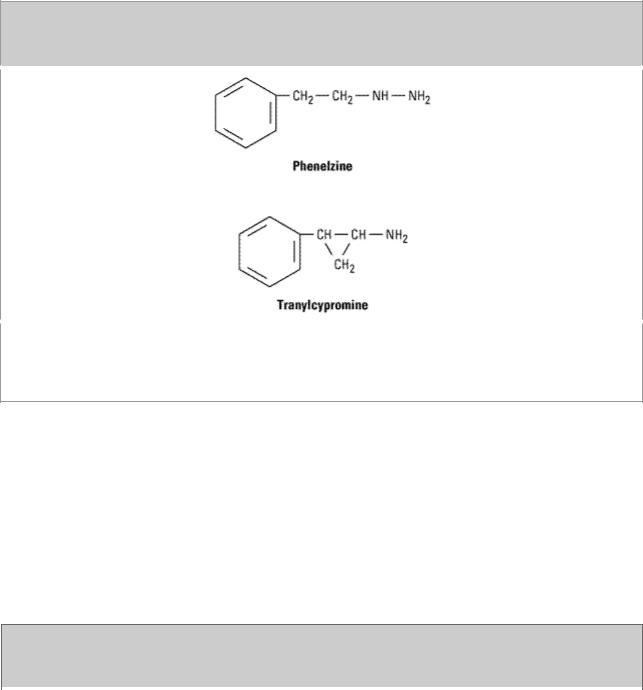
with phenelzine and isocarboxazid (no longer marketed); or nonhydrazides, which lack such a moiety, as with tranylcypromine (Figure 30–5). Tranylcypromine closely resembles dextroamphetamine, which is itself a weak inhibitor of MAO. Tranylcypromine retains some of the sympathomimetic characteristics of the amphetamines. The hydrazides appear to combine irreversibly with the enzyme, while tranylcypromine has a prolonged duration of effect even though it is not bound irreversibly. These older MAO inhibitors are nonselective inhibitors of both MAO-A and MAO-B.
Figure 30–5.
Some monoamine oxidase inhibitors. Phenelzine is the hydrazide of phenylethylamine (Figure 9– 3), while tranylcypromine has a cyclopropyl amine side chain and closely resembles dextroamphetamine (see Figure 9–4). These agents are unselective and produce an extremely long-lasting inhibition of the enzyme.
Pharmacokinetics
Tricyclics
Most tricyclics are incompletely absorbed and undergo significant first-pass metabolism. As a result of high protein binding and relatively high lipid solubility, volumes of distribution tend to be very large. Tricyclics are metabolized by two major routes: transformation of the tricyclic nucleus and alteration of the aliphatic side chain. Monodemethylation of tertiary amines leads to active metabolites such as desipramine and nortriptyline (which are themselves available as drugs; Figure 30–1). The pharmacokinetic parameters of various antidepressants are summarized in Table 30–2.
Table 30–2. Pharmacokinetic Parameters of Various Antidepressants.1,2
Drug |
Bioavailability |
Protein |
Plasma |
Active |
Volume of |
Therapeutic |
|
(percent) |
Binding |
t1/2 |
Metabolites |
Distribution |
Plasma |
|
|
(percent) |
(hours) |
|
(L/kg) |
Concentrations |
|
|
|
|
|
|
(ng/mL) |
|
|
|
|
|
|
|
Amitriptyline |
31–61 |
82–96 |
31–46 |
Nortriptyline |
5–10 |
80–200 total |
|
|
|
|
|
|
|

Amoxapine |
nd |
nd |
8 |
7-,8-Hydroxy |
nd |
nd |
|
|
|
|
|
|
|
Bupropion |
60–80 |
85 |
14–37 |
Hydroxy, |
20–30 |
25–100 |
|
|
|
|
threohydro, |
|
|
|
|
|
|
erythreohydro |
|
|
|
|
|
|
|
|
|
Citalopram |
51–93 |
70–80 |
23–75 |
Desmethyl |
12–16 |
nd |
|
|
|
|
|
|
|
Clomipramine |
nd |
nd |
22–84 |
Desmethyl |
7–20 |
240–700 |
|
|
|
|
|
|
|
Desipramine |
60–70 |
73–90 |
14–62 |
Hydroxy |
22–59 |
> 125 |
|
|
|
|
|
|
|
Doxepin |
13–45 |
nd |
8–24 |
Desmethyl |
9–33 |
30–150 |
|
|
|
|
|
|
|
Escitalopram |
80 |
56 |
27–59 |
5-Desmethyl |
12 |
nd |
|
|
|
|
|
|
|
Fluoxetine |
70 |
94 |
24–96 |
Norfluoxetine |
12–97 |
nd |
|
|
|
|
|
|
|
Fluvoxamine |
> 90 |
77 |
7–63 |
None |
> 5 |
nd |
|
|
|
|
|
|
|
Imipramine |
29–77 |
76–95 |
9–24 |
Desipramine |
15–30 |
> 180 total |
|
|
|
|
|
|
|
Maprotiline |
66–75 |
88 |
21–52 |
Desmethyl |
15–28 |
200–300 |
|
|
|
|
|
|
|
Mirtazapine |
nd |
nd |
20–40 |
Desmethyl |
nd |
nd |
|
|
|
|
|
|
|
Nefazodone |
15–23 |
98 |
2–4 |
Hydroxy, m- |
nd |
nd |
|
|
|
|
chlorophenyl |
|
|
|
|
|
|
piperazine |
|
|
|
|
|
|
|
|
|
Nortriptyline |
32–79 |
93–95 |
18–93 |
10-Hydroxy |
21–57 |
50–150 |
|
|
|
|
|
|
|
Paroxetine |
50 |
95 |
24 |
None |
28–31 |
nd |
|
|
|
|
|
|
|
Protriptyline |
77–93 |
90–95 |
54–198 |
nd |
19–57 |
70–170 |
|
|
|
|
|
|
|
Sertraline |
nd |
98 |
22–35 |
Desmethyl |
20 |
nd |
|
|
|
|
|
|
|
Trazodone |
nd |
nd |
4–9 |
m-Chloro- |
nd |
nd |
|
|
|
|
phenyl- |
|
|
|
|
|
|
piperazine |
|
|
|
|
|
|
|
|
|
Venlafaxine |
nd |
27–30 |
4–10 |
O-Desmethyl |
nd |
nd |
|
|
|
|
|
|
|
1Range includes active metabolites.
2nd = no data found.
Heterocyclics
The pharmacokinetics of these drugs are similar to those of the tricyclics (Table 30–2). Some may have active metabolites. Trazodone and venlafaxine have short plasma half-lives, which mandates divided doses during the day when beginning treatment, though once-a-day dosing may be possible later. Extended-release forms of bupropion and venlafaxine allow for once-a-day dosing in some patients from the outset.
Selective Serotonin Reuptake Inhibitors (SSRIs)
The pharmacokinetic parameters of these drugs are summarized in Table 30–2. Fluoxetine is notable for the long half-life of its active metabolite, norfluoxetine (7–9 days at steady state). This long t 1/2 has allowed for the introduction of a formulation for once-weekly dosing. Fluoxetine

inhibits various drug-metabolizing enzymes, which has led to a number of significant drug-drug interactions with other antidepressants and with other drugs as well. Sertraline and paroxetine have pharmacokinetic parameters similar to those of tricyclics. Citalopram and fluvoxamine resemble fluoxetine.
MAO Inhibitors
The monoamine oxidase inhibitors (MAOIs) are readily absorbed from the gastrointestinal tract. The hydrazide inhibitor phenelzine is acetylated in the liver and manifests differences in elimination depending on the acetylation phenotype of the individual (see Chapter 4: Drug Biotransformation). However, inhibition of MAO persists even after these drugs are no longer detectable in plasma. Therefore, conventional pharmacokinetic parameters (half-life, etc) are not very helpful in governing dosage. It is prudent to assume that the drug effect will persist for from 7 days (tranylcypromine) to 2 or 3 weeks (phenelzine) after discontinuance of the drug.
Pharmacodynamics
Action of Antidepressants on Biogenic Amine Neurotransmitters
The amine hypothesis was buttressed by studies on the mechanism of action of various types of antidepressant drugs (Figure 30–6). Tricyclics block the amine (norepinephrine or serotonin) reuptake pumps, which terminate amine neurotransmission (see Table 30–3 and Chapter 6: Introduction to Autonomic Pharmacology). Such an action presumably permits a longer sojourn of neurotransmitter at the receptor site. MAO inhibitors block a major degradative pathway for the amine neurotransmitters, which permits more amines to accumulate in presynaptic stores and more to be released. Some of the second-generation antidepressants have similar effects on amine neurotransmitters, while others have mild or minimal effects on reuptake or metabolism. In contrast, trazodone, nefazodone, and mirtazapine stand out as agents in which antagonism of subtypes of serotonin receptors (5-HT2A or 5-HT2C) may be important in their action. Mirtazapine is unique in including antagonism of  2 norepinephrine receptors as presumably contributing to its therapeutic effects. Bupropion has been found to alter the output of norepinephrine in humans following chronic administration through some as yet unidentified primary mechanism as well as occupying about 25% of dopamine uptake pumps in the brain as revealed by positron emission tomography. Since it has been shown that effective doses of SSRIs occupy 80% of serotonin uptake sites, the clinical relevance of 25% dopamine uptake occupancy is uncertain. Thus, even the newest antidepressants can still be categorized as working through serotonergic and noradrenergic effects with the possibility of a role for dopamine. A potential dopaminergic mechanism has often been invoked as relevant to the efficacy of MAOIs.
2 norepinephrine receptors as presumably contributing to its therapeutic effects. Bupropion has been found to alter the output of norepinephrine in humans following chronic administration through some as yet unidentified primary mechanism as well as occupying about 25% of dopamine uptake pumps in the brain as revealed by positron emission tomography. Since it has been shown that effective doses of SSRIs occupy 80% of serotonin uptake sites, the clinical relevance of 25% dopamine uptake occupancy is uncertain. Thus, even the newest antidepressants can still be categorized as working through serotonergic and noradrenergic effects with the possibility of a role for dopamine. A potential dopaminergic mechanism has often been invoked as relevant to the efficacy of MAOIs.
Figure 30–6.
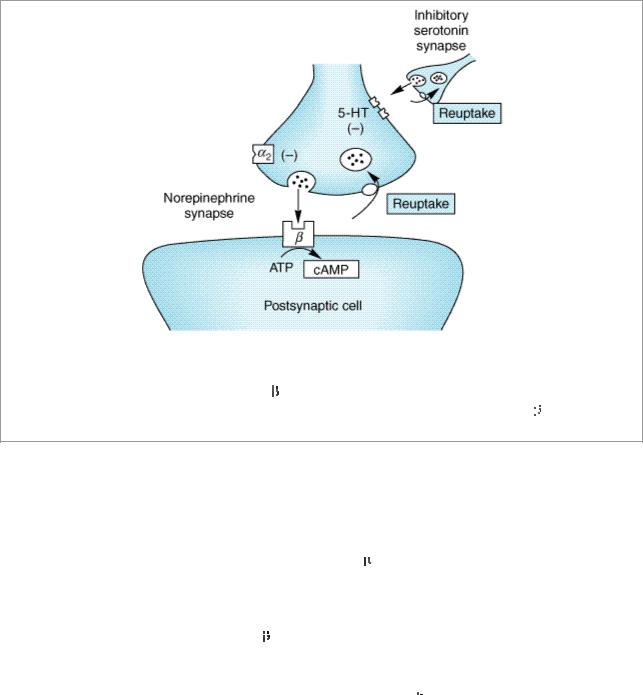
Schematic diagram showing some of the potential sites of action of antidepressant drugs. Chronic therapy with these drugs has been proved to reduce reuptake of norepinephrine or serotonin (or both), reduce the number of postsynaptic 
 receptors, and reduce the generation of cAMP. The MAO inhibitors act on MAO in the nerve terminals and cause the same effects on
receptors, and reduce the generation of cAMP. The MAO inhibitors act on MAO in the nerve terminals and cause the same effects on 
 receptors and cAMP generation.
receptors and cAMP generation.
Receptor and Postreceptor Effects
Considerable attention has been paid to the ultimate postsynaptic effects of increased neurotransmitters in the synapses. In tests of postsynaptic effects, cAMP concentrations have consistently decreased rather than increased, in spite of the presumably longer duration of action of the transmitters. In addition, the number of postsynaptic 
 -adrenoceptors has shown a measurable decrease that follows the same delayed time course as clinical improvement in patients. Thus, the initial increase in neurotransmitter seen with some antidepressants appears to produce, over time, a compensatory decrease in receptor activity, ie, down-regulation of receptors. Decreases in norepinephrine-stimulated cAMP and in
-adrenoceptors has shown a measurable decrease that follows the same delayed time course as clinical improvement in patients. Thus, the initial increase in neurotransmitter seen with some antidepressants appears to produce, over time, a compensatory decrease in receptor activity, ie, down-regulation of receptors. Decreases in norepinephrine-stimulated cAMP and in  -adrenoceptor binding have been conclusively shown for selective norepinephrine uptake inhibitors, those with mixed action on norepinephrine and serotonin, monoamine oxidase inhibitors, and even electroconvulsive therapy. Such changes do not consistently occur after the selective serotonin uptake inhibitors,
-adrenoceptor binding have been conclusively shown for selective norepinephrine uptake inhibitors, those with mixed action on norepinephrine and serotonin, monoamine oxidase inhibitors, and even electroconvulsive therapy. Such changes do not consistently occur after the selective serotonin uptake inhibitors, 
 2 receptor antagonists, and mixed serotonin antagonists.
2 receptor antagonists, and mixed serotonin antagonists.
It has also been emphasized that enhanced serotonergic transmission, albeit mediated through diverse mechanisms, might be a common (but not universal) long-term effect of antidepressants. This could occur, for instance, without increasing the concentration of neurotransmitter at the receptor site if there were an increase in serotonin receptor sensitivity. Thus, chronic treatment with tricyclic agents or electroconvulsive shock increases the electrophysiologic response to microiontophoretically applied serotonin in various areas of the rat brain. And selective receptor antagonism of either norepinephrine or serotonin receptors may lead to enhanced extracellular serotonin due to the complex manner in which these neurotransmitters are interregulated. One current hypothesis holds that enhanced stimulation or responsiveness of postsynaptic 5-HT1A receptors is particularly important in the action of antidepressants.

Most recently, long-term intracellular changes involving phosphorylation of various regulatory elements, including those within the nucleus, have been implicated as relevant to antidepressant action. It is possible that effects on certain neurotrophic factors—factors critical to sustained survival and function of neurons in the adult nervous system—may be central to the actions of antidepressants.
No clinical studies have directly tested the relevance of these findings from animals for norepinephrine and serotonin function in humans and their relationship to the mode of action of antidepressants. One of the most interesting approaches has been to reduce the amino acid precursor of serotonin, tryptophan, in the diet and, by implication, the amount of available neurotransmitter in the brain, since tryptophan availability can be rate-limiting in the formation of serotonin under certain experimental conditions. Using this approach, it was found that a tryptophan-depleted diet produces low plasma tryptophan and acutely reverses antidepressant responses to SSRIs but not to selective or mixed norepinephrine uptake inhibitors. Similarly, depletion of the norepinephrine amino acid precursor tyrosine can reverse antidepressant effects of the relatively selective norepinephrine reuptake inhibitor, desipramine. These findings indirectly support the hypothesis that enhanced serotonin throughput is necessary for the antidepressant action of serotonin but not norepinephrine uptake inhibitors. The same appears to be true of norepinephrine throughput and norepinephrine reuptake inhibitors. However, tryptophan depletion does not consistently worsen the condition of unmedicated depressed patients. Thus, there is no clear relationship between serotonin and depression or antidepressant mechanisms in general.
Effects of Specific Antidepressants
Tricyclics
The first-generation antidepressants demonstrate varying degrees of selectivity for the reuptake pumps for norepinephrine and serotonin (Table 30–3). They also have numerous autonomic actions, as described below under Adverse Effects.
Table 30–3. Pharmacologic Differences among Several Antidepressants.1
|
|
|
|
Block of Amine Pump for: |
|
|
|
Drug |
Sedative |
Antimuscarinic |
|
Serotonin |
Norepinephrine |
Dopamine |
|
|
Action |
Action |
|
|
|
|
|
Amitriptyline |
+++ |
+++ |
+++ |
++ |
0 |
|
|
|
|
|
|
|
|
|
|
Amoxapine |
++ |
++ |
+ |
++ |
+ |
|
|
Bupropion |
0 |
0 |
+, 0 |
+, 0 |
+ |
|
|
|
|
|
|
|
|
|
|
Citalopram, |
0 |
0 |
+++ |
0 |
0 |
|
|
escitalopram |
|
|
|
|
|
|
|
Clomipramine |
+++ |
++ |
+++ |
+++ |
0 |
|
|
|
|
|
|
|
|
|
|
Desipramine |
+ |
+ |
0 |
+++ |
0 |
|
|
|
|
|
|
|
|
|
|
Doxepin |
+++ |
+++ |
++ |
+ |
0 |
|
|
Fluoxetine |
+ |
+ |
+++ |
0, + |
0, + |
|
|
|
|
|
|
|
|
|
|
Fluvoxamine |
0 |
0 |
+++ |
0 |
0 |
|
|
|
|
|
|
|
|
|
|

Imipramine |
|
++ |
++ |
+++ |
++ |
|
0 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
Maprotiline |
|
++ |
++ |
0 |
|
+++ |
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mirtazapine2 |
|
+++ |
0 |
0 |
|
0 |
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nefazodone |
|
++ |
+++ |
+, 0 |
|
0 |
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nortriptyline |
|
++ |
++ |
+++ |
|
++ |
|
|
0 |
|
|
Paroxetine |
|
+ |
0 |
+++ |
|
0 |
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Protriptyline |
|
0 |
++ |
? |
|
+++ |
|
|
? |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sertraline |
|
+ |
0 |
+++ |
|
0 |
|
|
0 |
|
|
Trazodone |
|
+++ |
0 |
++ |
|
0 |
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Venlafaxine |
|
0 |
0 |
+++ |
|
++ |
|
|
0, + |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 = none; + = slight; ++ = moderate; +++ = high; ? = uncertain. |
|
|
|
|
|
|
|
||||
2Significant |
2-adrenoceptor antagonism. |
|
|
|
|
|
|
|
|
||
Second-Generation Agents |
|
|
|
|
|
|
|
|
|
||
Amoxapine is a metabolite of the antipsychotic drug loxapine and retains some of its antipsychotic action and dopamine receptor antagonism. A combination of antidepressant and antipsychotic actions might make it a suitable drug for depression in psychotic patients. However, the dopamine antagonism may cause akathisia, parkinsonism, amenorrhea-galactorrhea syndrome, and perhaps tardive dyskinesia.
Maprotiline (a tetracyclic drug) is most like desipramine in terms of its potent norepinephrine uptake inhibition. Like the latter drug, it has fewer sedative and antimuscarinic actions than the older tricyclics.
Clinical experience with trazodone has indicated unpredictable efficacy for depression though it has proved very useful as a hypnotic, sometimes being combined with MAOIs, which disturb sleep.
Third-Generation Agents
Three antidepressants—nefazodone, venlafaxine, and mirtazapine—are all related to earlier agents in either structure or mechanism of action. Nefazodone is closely related to trazodone but is less sedating. It produces fewer adverse sexual effects than the SSRIs but is a potent inhibitor of CYP3A4. (Fluvoxamine causes the same inhibition of CYP3A4.)
Venlafaxine is a potent inhibitor of serotonin reuptake and a weaker inhibitor of norepinephrine transport such that at lower therapeutic doses it behaves like an SSRI. At high doses (more than 225 mg/d) it produces mild to moderate increases of heart rate and blood pressure attributable to norepinephrine reuptake inhibition. Doses in the range of 300 mg/d or greater may confer broader therapeutic effects than SSRIs, but a careful titration up to these doses is needed to control adverse effects.
Mirtazapine, a drug derived from mianserin—an antidepressant available outside the USA—is a
