
Reactive Intermediate Chemistry
.pdf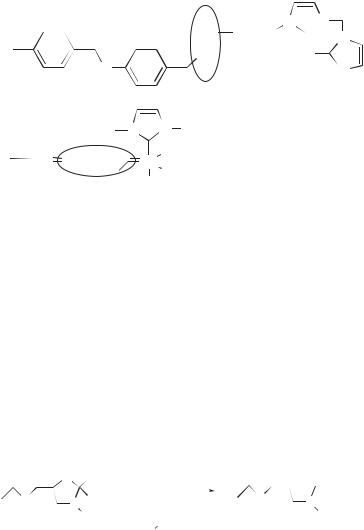
364 STABLE SINGLET CARBENES
(a)
|
|
|
|
|
|
N |
|
N |
|
|
|
|
|
|
HO |
(CH2)n |
|
N |
|
|
|
|
|||||||
|
|
|
|
|
Br |
Br |
|
Pd |
|
|
|
|
|
|
|
||||
|
|
|
O |
|
|
|
|
||
|
|
|
|
|
Br N |
||||
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
H |
(b)
|
Ar |
N N Ar |
|
H |
Cl |
|
CH CH2 |
|
|
Ru |
|
|
R |
Cl |
|
PR3 |
|
|
|
|
|
|
Scheme 8.29 |
unclear’’. . . and that ‘‘these developments illustrate the extent to which it is possible to tune a metal center by modifying the ligand environment’’.103
Maximizing recovery of the catalyst is a key issue, especially because residual metal may cause decomposition of the product over time and increase toxicity of the final material. The NHC are particularly suitable ligands for immobilization, since as already mentioned they are strongly bound to the metals, do not undergo dissociation during the catalytic process, and are remarkably air and water stable. The first polymer-supported NHC catalysts were reported in 2000.137 The synthetic strategies can be divided into two types. Either the transition metal catalyst is first synthesized and then grafted to the support (Scheme 8.29), or alternatively a carbene precursor is first grafted to the support and only then is the carbene generated and coordinated to the metal (Scheme 8.30).
|
|
Ar |
|
|
|
|
|
|
|
|
|
|
Ar |
|
CHPh |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
N |
H |
1) ∆ (−t-BuOH) |
|
|
N |
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
Ru |
|
PR3 |
||
|
O |
|
N |
Ot-Bu |
2) Cl |
|
|
PR3 |
|
|
NCl |
|
|
|
Cl |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
Ar |
|
|
|
Ar |
||||||||||||||
|
|
|
|
Cl |
Ru |
|
CHPh |
|
|
||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
PR3 |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Scheme 8.30
Using the first approach, Herrmann and co-workers137a obtained goods results in the Heck coupling reactions, using palladium complexes grafted on the Wang resin via one of the amino substituents of the NHC. As expected, hardly any catalyst leaching was observed during the catalytic reactions (Scheme 8.29a).
Hoveyda and co-workers137b immobilized an olefin metathesis catalyst on monolithic sol–gel and claimed that the catalytic material is easily recyclable. Barrett and co-workers137c prepared a ‘‘recyclable boomerang polymer supported catalyst for olefin methathesis’’ by grafting the preformed catalyst to a polystyrene
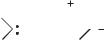
CONCLUSION AND OUTLOOK |
365 |
resin. Very elegantly, the ‘‘classical’’ carbene that initiates the metathesis is itself anchored to the vinylated polystyrene resin by a metathetical step (Scheme 8.29b). Note that in this case the NHC is not directly bound to the support and thus the precatalyst becomes soluble during the course of the reaction and must be recaptured by the polymer.
The alternative strategy for heterogenization has been pursued by Blechert and co-workers,137d for a polymer-supported olefin metathesis catalyst. A polymeranchored carbene precursor was prepared by coupling an alkoxide to a cross-linked polystyrene Merrifield-type resin. Subsequently, the desired polymer-bound carbene complex was formed by thermolytically induced elimination of tert-butanol while heating the precursor resin in the presence of the desired transition metal fragment (Scheme 8.30).
Similarly, Buchmeister and co-workers137e functionalized a monolithic carrier by an imidazolium carbene precursor and, following generation of the carbene by deprotonation, prepared the active ruthenium catalyst.
6. CONCLUSION AND OUTLOOK
From this chapter, it is clear that the common statement that carbenes only occur as transient reactive intermediates is no longer valid.
Due the nature of the substituents, all the stable singlet carbenes exihibit some carbon-heteroatom multiple-bond character and for some time their carbene nature has been a subject of controversy. One has to keep in mind that apart from dialkylcarbenes, all the transient singlet carbenes present similar electronic interactions. As early as 1956, Skell and Garner138 drew the transient dibromocarbene in its ylide form based on ‘‘the overlap of the vacant p-orbital of carbon with the filled p orbitals of the bromine atoms’’ (Scheme 8.31).
Br Br
Br Br
Scheme 8.31
Up to 2000, the number and variety of stable carbenes have been limited by the perceived necessity for two strongly interacting substituents. The preparation of stable or persistent (aryl)- or (alkyl)-(phosphino)carbenes as well as (aryl)(amino)- carbenes demonstrates that a single electron-active substituent allows the spectroscopic characterization of singlet carbenes under standard laboratory conditions. It has been shown that an amino substituent is more efficient than a phosphino substituent to stabilize a carbene center and that the steric bulk of the spectator substituent can be decreased even to the size of a methyl group in the phosphino series, so that a broad range of ‘‘observable’’ carbenes will be readily available.
366 STABLE SINGLET CARBENES
In the last couple of years, it has been demonstrated that NHC strongly bind transition metal centers, leading to extremely active and robust catalysts, which often outperform their phosphine-based analogues. However, the structure of NHC can only be modified through variation in the nitrogen and carbon backbone substituents. Consequently, the steric demands of these ligands can easily be varied using the N-substituents, but their electronic character can only be modified slightly. Indeed, the only significant variation possible is a choice between using either a saturated or an unsaturated backbone.
The availability of stable carbenes featuring a broad range of substituents directly adjacent to the carbene center will allow for great variation of their electronic properties to be achieved and therefore catalytic activities of the resulting carbene complexes should be readily tuned.
Major breakthroughs can also be expected in asymmetric catalysis, because enantiomerically pure carbene complexes, with the source of asymmetry close to the metal center, will be accessible.
Stable carbenes are not laboratory curiosities any longer, they are powerful tools for a variety of chemists.
SUGGESTED READING
G.Bertrand, Ed., Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents, Marcel Dekker, New York, 2002.
W.A. Herrmann, ‘‘N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis,’’
Angew. Chem. Int. Ed. Engl. 2002, 41, 1290.
A.J. Arduengo, III and T. Bannenberg, ‘‘Nucleophilic Carbenes and their Applications in Modern Complex Catalysis,’’ The Strem Chemiker, 2002, XVIV, 2.
L.Jafarpour and S. P. Nolan, ‘‘Transition-Metal Systems Bearing a Nucleophilic Carbene Ancillary Ligand: From Thermochemistry to Catalysis,’’ Adv. Organomet. Chem. 2001, 46, 181.
D. Enders and H. Gielen, ‘‘Synthesis of Chiral Triazolinylidene and Imidazolinylidene Transition Metal Complexes and First Application in Asymmetric Catalysis,’’ J. Organomet. Chem. 2001, 617, 70.
T.M. Trnka and R. H. Grubbs, ‘‘The Development of L2X2Ru CHR Olefin Metathesis catalysts: An Organometallic Success Story,’’ Acc. Chem. Res. 2001, 34, 18.
D.Bourissou, O. Guerret, F. P. Gabbaı¨, and G. Bertrand, ‘‘Stable Carbenes,’’ Chem. Rev. 2000, 100, 39.
REFERENCES
1.J. B. Dumas and E. Peligot, Ann. Chim. Phys. 1835, 58, 5.
2.H. Staudinger and O. Kupfer, Ber. Dtsch. Chem. Ges. 1912, 45, 501.
3.R. Breslow, J. Am. Chem. Soc. 1958, 80, 3719.
4.H.-W. Wanzlick, Angew. Chem. 1962, 74, 129.
REFERENCES 367
5.(a) A. Igau, H. Grutzmacher, A. Baceiredo, and G. Bertrand, J. Am. Chem. Soc. 1988, 110, 6463. (b) A. Igau, A. Baceiredo, G. Trinquier, and G. Bertrand, Angew. Chem. Int. Ed. Engl. 1989, 28, 621.
6.(a) H. Tomioka, Acc. Chem. Res. 1997, 30, 315. (b) H. Tomioka, in Advances in Carbene Chemistry, Vol. 2, U. H. Brinker, Ed., JAI Press, Stamford, 1998, p. 175. (c) H. Tomioka, E. Iwamoto, H. Takura, and K. Hirai, Nature (London) 2001, 412, 626. (d) H. Tomioka, T. Watanabe, M. Hattori, N. Nomura, and K. Hirai, J. Am. Chem. Soc. 2002, 124, 474.
7.R. Gleiter and R. Hoffman, J. Am. Chem. Soc. 1968, 90, 1475.
8.B. C. Gilbert, D. Griller, and A. S. Nazran, J. Org. Chem. 1985, 50, 4738.
9.G. B. Schuster, Adv. Phys. Org. Chem. 1986, 22, 311.
10.D. R. Myers, V. P. Senthilnathan, M. S. Platz, and, J. Jones, Jr., J. Am. Chem. Soc. 1986, 108, 4232.
11.J. E. Gano, R. H. Wettach, M. S. Platz, and V. P. Senthilnathan, J. Am. Chem. Soc. 1982, 104, 2326.
12.(a) H. P. Reisenauer, G. Maier, A. Reimann, and R. W. Hoffmann, Angew. Chem. Int. Ed. Engl. 1984, 23, 641. (b) T. J. Lee, A. Bunge, and H. F. Schaefer, III, J. Am. Chem. Soc. 1985, 107, 137.
13.G. Xu, T. M. Chang, J. Zhou, M. L. McKee, and P. B. Shevlin, J. Am. Chem. Soc. 1999, 121, 7150.
14.(a) R. Hoffman, G. D. Zeiss, and G. W. Van Dine, J. Am. Chem. Soc. 1968, 90, 1485.
(b) N. C. Baird and K. F. Taylor, J. Am. Chem. Soc. 1978, 100, 1333.
15.(a) J. F. Harrison, J. Am. Chem. Soc. 1971, 93, 4112. (b) C. W. Bauschlicher, Jr., H. F. Schaefer, III, and P. S. Bagus, J. Am. Chem. Soc. 1977, 99, 7106. (c) J. F. Harrison, C. R. Liedtke, and J. F. Liebman, J. Am. Chem. Soc. 1979, 101, 7162. (d) D. Feller, W. T. Borden, and E. R. Davidson, Chem. Phys. Lett. 1980, 71, 22.
16.(a) W. W. Schoeller, Chem. Commun. 1980, 124. (b) L. Pauling, Chem. Commun. 1980, 688.
17.K. I. Irikura, W. A. Goddard, III, and J. L. Beauchamp, J. Am. Chem. Soc. 1992, 114, 48.
18.(a) R. A. Moss, M. Wlostowski, S. Shen, K. Krogh-Jespersen, and A. Matro, J. Am. Chem. Soc. 1988, 110, 4443. (b) X. M. Du, H. Fan, J. L. Goodman, M. A. Kesselmayer, K. Krogh-Jespersen, J. A. La Villa, R. A. Moss, S. Shen, and R. S. Sheridan, J. Am. Chem. Soc. 1990, 112, 1920.
19.(a) R. A. Mitsch, J. Am. Chem. Soc. 1965, 87, 758. (b) R. A. Moss and C. B. Mallon,
J. Am. Chem. Soc. 1975, 97, 344. (c) S. Koda, Chem. Phys. Lett. 1978, 55, 353.
20.(a) J. L. Wang, J. P. Toscano, M. S. Platz, V. Nikolaev, and V. Popik, J. Am. Chem. Soc. 1995, 117, 5477. (b) P. Visser, R. Zuhse, M. W. Wong, and C. Wentrup, J. Am. Chem. Soc. 1996, 118, 12598.
21.(a) A. Berndt, Angew. Chem. Int. Ed. Engl. 1993, 32, 985. (b) A. Berndt, D. Steiner, D. Schweikart, C. Balzereit, M. Menzel, J. H. Winkler, S. Mehle, M. Unverzagt, T. Happel, P. v. R. Schleyer, G. Subramanian, and M. Hofmann, in Advances in Boron Chemistry, W. Siebert, Ed., The Royal Society of Chemistry, Cambridge; 1997, p. 61. (c) M. Menzel, H. J. Winckler, T. Ablelom, D. Steiner, S. Fau, G. Frenking, W. Massa, and A. Berndt,
Angew. Chem. Int. Ed. Engl. 1995, 34, 1340.
22.R. A. Moss, C. B. Mallon, and C. T. Ho, J. Am. Chem. Soc. 1977, 99, 4105.
368STABLE SINGLET CARBENES
23.A. Baceiredo, G. Bertrand, and G. Sicard, J. Am. Chem. Soc. 1985, 107, 4781
24.Th. Curtius, Ber. Dtsch. Chem. Ges. 1889, 22, 2161.
25.(a) G. Sicard, A. Baceiredo, G. Bertrand, and J. P. Majoral, Angew. Chem. Int. Ed. Engl. 1984, 23, 459. (b) A. Baceiredo, G. Bertrand, J. P. Majoral, G. Sicard, J. Jaud, and J. Galy,
J.Am. Chem. Soc. 1984, 106, 6088.
26.For reviews: (a) R. W. Hoffmann, Angew. Chem. Int. Ed. Engl. 1968, 7, 754. (b) J. Hocker and R. Merten, Angew. Chem. Int. Ed. Engl. 1972, 11, 964.
27.For a review: N. Wiberg, Angew. Chem. Int. Ed. Engl. 1968, 7, 766.
28.D. M. Lemal, R. A. Lovald, and K. J. Kawano, J. Am. Chem. Soc. 1964, 86, 2518.
29.(a) H.-W. Wanzlick and H.-J. Schonherr, Liebigs Ann. Chem. 1970, 731, 176. (b) H.-J. Schonherr and H.-W. Wanzlick, Chem. Ber. 1970, 103, 1037.
30.A. J. Arduengo, III, R. L. Harlow, and M. Kline, J. Am. Chem. Soc. 1991, 113, 36.
31.A. J. Arduengo, III, J. R. Goerlich, R. Krafczyk, and W. J. Marshall, Angew. Chem. Int. Ed. Engl. 1998, 37, 1963.
32.For reviews: (a) G. Bertrand, in ‘‘Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents,’’ G., Bertrand Ed., Marcel Dekker, New York, 2002, p. 177.
(b) D. Bourissou, O. Guerret, F. P. Gabbaı¨, and G. Bertrand, Chem. Rev. 2000, 100, 39. (c) D. Bourissou and G. Bertrand, Advances in Organomet. Chem. 1999, 44, 175.
(d) R. Re´au and G. Bertrand, in Methoden der Organischen Chemie (Houben-Weyl),
M.Regitz, Ed., Georg Thieme Verlag: Stuttgart, 1996, E17a, p. 794. (e) G. Bertrand and R. Reed, Coord. Chem. Rev. 1994, 137, 323.
33.(a) M. Soleilhavoup, A. Baceiredo, O. Treutler, R. Ahlrichs, M. Nieger, and G. Bertrand,
J.Am. Chem. Soc. 1992, 114, 10959. (b) P. Dyer, A. Baceiredo, and G. Bertrand, Inorg. Chem. 1996, 35, 46.
34.T. Kato, H. Gornitzka, A. Baceiredo, A. Savin, and G. Bertrand, J. Am. Chem. Soc. 2000, 122, 998.
35.(a) J. Kapp, C. Schade, A. M. El-Nahasa, and P. v. R. Schleyer, Angew. Chem. Int. Ed. Engl. 1996, 35, 2236. (b) C. Schade and P. v. R. Schleyer, Chem. Commun. 1987, 1399.
36.S. Goumri, Y. Leriche, H. Gornitzka, A. Baceiredo, and G. Bertrand, Eur. J. Inorg. Chem. 1998, 1539.
37.(a) M. T. Nguyen, M. A. McGinn, and A. F. Hegarty, Inorg. Chem. 1986, 25, 2185.
(b) M. R. Hoffmann and K. Kuhler, J. Chem. Phys. 1991, 94, 8029. (c) D. A. Dixon, K. B. Dobbs, A. J. Arduengo, III, and G. Bertrand, J. Am. Chem. Soc. 1991, 113, 8782. (d) L. Nyulaszi, D. Szieberth, J. Reffy, and T. Veszpremi, J. Mol. Struct. (THEOCHEM) 1998, 453, 91.
38.C. Heinemann, T. Mu¨ller, Y. Apeloig, and H. Schwarz, J. Am. Chem. Soc. 1996, 118, 2023. C. Boehme and G. Frenking, J. Am. Chem. Soc. 1996, 118, 2039.
39.A. J., Arduengo, III, J. Goerlich, and W. Marshall, J. Am. Chem. Soc. 1995, 117, 11027.
40.R. W. Alder, M. E. Blake, C. Bortolotti, S. Bufali, C. P. Butts, E. Linehan, J. M. Oliva, A. G. Orpen, and M. J. Quayle, Chem. Commun. 1999, 241.
41.F. E. Hahn, L. Wittenbecher, R. Boese, and D. Bla¨ser, Chem. Eur. J. 1999, 5, 1931.
42.D. Enders, K. Breuer, G. Raabe, J. Runsink, J. H. Teles, J. P. Melder, K. Ebel, and S. Brode, Angew. Chem. Int. Ed. Engl. 1995, 34, 1021.
43.A. J. Arduengo, III, J. R. Goerlich, and W. J. Marshall, Liebigs Ann. 1997, 365.
REFERENCES 369
44.R. W. Alder, P. R. Allen, M. Murray, and G. Orpen, Angew. Chem. Int. Ed. Engl. 1996, 35, 1121.
45.R. W. Alder, C. P. Butts, and A. G. Orpen, J. Am. Chem. Soc. 1998, 120, 11526.
46.(a) W. A. Herrmann, M. Elison, J. Fischer, C. Ko¨cher, and G. R. J. Artus, Chem. Eur. J. 1996, 2, 772. (b) W. A. Herrmann, C. Ko¨cher, L. J. Goossen, and G. R. J. Artus, Chem. Eur. J. 1996, 2, 1627.
47.N. Kuhn and T. Kratz, Synthesis 1993, 561.
48.D. A. Dixon and A. J. Arduengo, III, J. Phys. Chem. 1991, 95, 4180.
49.(a) A. J. Arduengo, III, D. A. Dixon, K. K. Kumashiro, C. Lee, W. P. Power, and K. W. Zlim, J. Am. Chem. Soc. 1994, 116, 6361. (b) A. J. Arduengo, III, H. V. R. Dias, D. A. Dixon, R. L. Harlow, W. T. Klooster, and T. F. Koetzle, J. Am. Chem. Soc. 1994, 116, 6812. (c) A. J. Arduengo, III, H. Bock, H. Chen, M. Denk, D. A. Dixon, J. C. Green,
W.A. Herrmann, N. L. Jones, M. Wagner, and R. West, J. Am. Chem. Soc. 1994, 116, 6641.
50.J. Cioslowski, Int. J. Quantum Chem., Quant. Chem. Symp. 1993, 27, 309.
51.(a) C. Heinemann, T. Mu¨ller, Y. Apeloig, and H. Schwarz, J. Am. Chem. Soc. 1996, 118, 2023. (b) C. Boehme and G. Frenking, J. Am. Chem. Soc. 1996, 118, 2039.
52.J. F. Lehmann, S. G. Urquhart, L. E. Ennis, A. P. Hitchcock, K. Hatano, S. Gupta, and
M.K. Denk, Organometallics 1999, 18, 1862.
53.R. W. Alder and M. E. Blake, Chem. Commun. 1997, 1513.
54.R. W. Alder, M. E. Blake, and J. M. Oliva, J. Phys. Chem. A 1999, 103, 11200.
55.(a) R. A. Moss, T. Zdrojewski, and G. Ho, Chem. Commun. 1991, 946. (b) D. L. S. Brahms and W. P. Dailey, Chem. Rev. 1996, 96, 1585.
56.C. Buron, H. Gornitzka, V. Romanenko, and G. Bertrand, Science 2000, 288, 834.
57.(a) J. E. Jackson and M. S. Platz, Adv. Carbene Chem. 1994, 1, 89. (b) A. Admasu, A. D. Gudmundsdottir, M. S. Platz, D. S. Watt, S. Swiatkowski, and P. J. Crocker, J. Chem. Soc., Perkin Trans. 2 1998, 1093.
58.(a) R. T. Ruck and M. Jones, Jr., Tetrahedron Lett. 1998, 39, 2277. (b) K. KroghJespersen, S. Yan, and R. A. Moss, J. Am. Chem. Soc. 1999, 121, 6269. (c) R. A. Moss,
S.Yan, and K. Krogh-Jespersen, J. Am. Chem. Soc. 1998, 120, 1088. (d) M. I. Khan and
J.L. Goodman, J. Am. Chem. Soc. 1995, 117, 6635.
59.W. W. Schoeller, Eur. J. Org. Chem. 2000, 2, 369.
60.S. Sole, H. Gornitzka, W. W. Schoeller, D. Bourissou, and G. Bertrand, Science 2001, 292, 1901.
61.K. Hirai, K. Komatsu, and H. Tomioka, Chem. Lett. 1994, 503.
62.H. Tomioka and K. Taketsuji, Chem. Commun. 1997, 1745.
63.E. Despagnet, H. Gornitzka, A. B. Rozhenko, W. W. Schoeller, D. Bourissou, and
G.Bertrand, Angew. Chem. Int. Ed. Engl. 2002, 41, 2835.
64.U. H. Brinker and M. G. Rosenberg, in Advances in Carbene Chemistry, Vol. 2, U. H. Brinker, Ed., JAI Press, Stamford, 1998, p. 29.
65.R. S. Sheridan, R. A. Moss, B. K. Wilk, S. Shen, M. Wlostowski, M. A. Kesselmayer,
R.Subramanian, G. Kmiecik-Lawrynowicz, and K. Krogh-Jespersen, J. Am. Chem. Soc. 1988, 110, 7563.
66.R. Bonneau and M. T. H. Liu, in Advances in Carbene Chemistry, Vol. 2, U. H. Brinker, Ed., JAI Press, Stamford, 1998, p. 1.
370STABLE SINGLET CARBENES
67.N. Merceron, K. Miqueu, A. Baceiredo, and G. Bertrand. J. Am. Chem. Soc. 2002, 124, 6806.
68.W. A. Herrmann, Angew. Chem. Int. Ed. Engl. 2002, 41, 1290.
69.(a) H.-W. Wanzlick and H.-J. Kleiner, Angew. Chem. 1961, 73, 493. (b) M. K. Denk,
K.Hatano, and M. Ma, Tetrahedron Lett. 1999, 40, 2057.
70.Y. F. Liu and D. M. Lemal, Tetrahedron Lett. 2000, 41, 599.
71.E. A. Carter and W. A. Goddard, III, J. Phys. Chem. 1986, 90, 998.
72.C. Heinemann and W. Thiel, Chem. Phys. Lett. 1994, 217, 11.
73.T. A. Taton and P. Chen, Angew. Chem. Int. Ed. Engl. 1996, 35, 1011.
74.G. Raabe, K. Breuer, D. Enders, and J. H. Teles, Z. Naturforsch. 1996, 51a, 95.
75.(a) G. Trinquier and J. P. Malrieu, J. Am. Chem. Soc. 1987, 109, 5303. (b) J. P. Malrieu and G. Trinquier, J. Am. Chem. Soc. 1989, 111, 5916. (c) H. Jacobsen and T. Ziegler,
J.Am. Chem. Soc. 1994, 116, 3667.
76.R. Alder, in ‘‘Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents,’’
G.Bertrand, Ed., Marcel Dekker, New York, 2002, p. 153.
77.(a) M. K. Denk, A. Thadani, K. Hatano, and A. J. Lough, Angew. Chem. Int. Ed. Engl. 1997, 36, 2607. (b) Z. Shi, V. Goulle, and R. P. Thummel, Tetrahedron Lett. 1996, 37, 2357.
78.Y.-T. Chen and F. Jordan, J. Org. Chem. 1991, 56, 5029.
79.(a) R. W. Alder, P. R. Allen, and S. J. Williams, Chem. Commun. 1995, 1267. (b) Y.-J. Kim and A. Streitwieser, J. Am. Chem. Soc. 2002, 124, 5757.
80.For a particularly clear example of this effect, the reader is referred to Ref. 58a.
81.(a) R. A. Moss, Acc. Chem. Res. 1989, 22, 15. (b) Carbocyclic Three-Membered Ring Compounds, Vol. E17a. Methoden der Organische Chemie (Houben-Weyl),
A.de Meijere, Ed., Thieme Verlag, Stuttgart, 1996.
82.(a) M. Brookhart and W. B. Studabaker, Chem. Rev. 1987, 87, 411. (b) D. F. Harvey and
D.M. Sigano, Chem. Rev. 1996, 96, 271. (c) H. W. Fru¨hauf, Chem. Rev. 1997, 97, 523.
83.P. S. Skell, Tetrahedron 1985, 41, 1427.
84.S. Goumri-Magnet, T. Kato, H. Gornitzka, A. Baceiredo, and G. Bertrand, J. Am. Chem. Soc. 2000, 122, 4464.
85.(a) A. E. Keating, S. R. Merrigan, D. A. Singleton, and K. N. Houk, J. Am. Chem. Soc. 1999, 121, 3933. (b) N. G. Rondan, K. N. Houk, and R. A. Moss, J. Am. Chem. Soc. 1980, 102, 1770.
86.G. Alcaraz, U. Wecker, A. Baceiredo, F. Dahan, and G. Bertrand, Angew. Chem. Int. Ed. Engl. 1995, 34, 1246. V. Piquet, A. Baceiredo, H. Gornitzka, F. Dahan, and G. Bertrand,
Chem. Eur. J. 1997, 3, 1757.
87.M. Sanchez, R. Re´au, C. J. Marsden, M. Regitz, and G. Bertrand, Chem. Eur. J. 1999, 5,
274.R. Armbrust, M. Sanchez, R. Re´au, U. Bergstrasser, M. Regitz, and G. Bertrand,
J.Am. Chem. Soc. 1995, 117, 10785.
88.G. Bertrand, Angew. Chem. Int. Ed. Engl. 1998, 37, 270.
89.(a) H. J. Bestmann and R. Zimmermann, in Methoden der Organischen Chemie
(Houben-Weyl), M. Regitz, Ed., Georg Thieme Verlag, Stuttgart, 1982, Vol. E1, p. 616. (b) A. W. Johnson, W. C. Kaska, K. A. O. Starzewski, and D. A. Dixon, in Ylides and Imines of Phosphorus, John Wiley & Sons, Inc., New York, 1993, p. 115.
REFERENCES 371
90.S. Goumri-Magnet, O. Polischuck, H. Gornitzka, C. J. Marsden, A. Baceiredo, and
G.Bertrand, Angew. Chem. Int. Ed. Engl. 1999, 38, 3727.
91.(a) A. J. Arduengo, III, H. V. R. Dias, and J. C. Calabrese, Chem. Lett. 1997, 143.
(b) A. J. Arduengo, III, J. C. Calabrese, A. H. Cowley, H. V. R. Dias, J. R. Goerlich,
W.J. Marshall, and B. Riegel, Inorg. Chem. 1997, 36, 2151.
92.N. Kuhn, J. Fahl, D. Blaser, and R. Boese, Z. Anorg. Allg. Chem. 1999, 625, 729.
93.A. J., Arduengo, III, M. Kline, J. C. Calabrese, and F. Davidson, J. Am. Chem. Soc. 1991, 113, 9704.
94.N. Kuhn, T. Kratz, and G. Henkel, Chem. Commun. 1993, 1778.
95.N. Kuhn, H. Bohnen, D. Bla¨ser, R. Boese, and A. H. Maulitz, Chem. Commun. 1994, 2283.
96.A. J. Arduengo, III, H. V. R. Dias, J. C. Calabrese, and F. Davidson, J. Am. Chem. Soc. 1992, 114, 9724.
97.D. E. Hibbs, M. B. Hursthouse, C. Jones, and N. A. Smithies, Chem. Commun. 1998, 869.
98.S. J. Black, D. E. Hibbs, M. B. Hursthouse, C. Jones, K. M. A. Malik, and N. A. Smithies,
J.Chem. Soc., Dalton Trans. 1997, 4313.
99.A. Cowley, F. Gabbaı¨, C. Carrano, L. Mokry, M. Bond, and G. Bertrand, Angew. Chem. Int. Ed. Engl. 1994, 33, 578.
100.K. H. v. Locquenghien, A. Baceiredo, R. Boese, and G. Bertrand, J. Am. Chem. Soc. 1991, 113, 5062.
101.W. A. Herrmann and C. Ko¨cher, Angew. Chem. Int. Ed. Engl. 1997, 36, 2163.
102.L. Jafarpour and S. P. Nolan, Adv. Organomet. Chem. 2001, 46, 181.
103.T. M. Trnka and R. H. Grubbs, Acc. Chem. Res. 2001, 34, 18.
104. |
¨ |
¨ |
(a) K. Ofele, J. Organomet. Chem. 1968, 12, P42. (b) K. Ofele and C. G. Kreiter, Chem. |
||
|
Ber. 1972, 105, 529. |
|
105. |
D. J. Cardin, B. Cetinkaya, and M. F. Lappert, Chem. Rev. 1972, 72, 545. |
|
106. |
(a) E. Peris, J. A. Loch, J. Mata, and R. H. Crabtree, Chem. Commun. 2001, 201. (b) M. |
|
|
Albrecht, J. R. Miecznikowski, A. Samuel, J. W. Faller, and R. H. Crabtree, Organo- |
|
|
metallics 2002, 21, 3596. (c) M. S. Viciu, R. M. Kissling, E. D. Stevens, and S. P. Nolan, |
|
|
Org. Lett. 2002, 4, 2229. |
|
107. |
I. Huang, H.-J. Schanz, E. D. Stevens, and S. P. Nolan, Organometallics 1999, 18, 2370. |
|
108. |
(a) K. Denk, P. Sirsch, and W. A. Herrmann, J. Organomet. Chem. 2002, 649, 219. (b) M. |
|
|
Tafipolsky, M. Scherer, K. Ofele, G. Artus, B. Pedersen, W. A. Herrmann, and S. G. |
|
|
McGrady, J. Am. Chem. Soc. 2002, 124, 5865. |
|
109. |
For attempted coordination of an anionic di(phosphino)carbene, see: A. Fuchs, D. Gudat, |
|
|
M. Nieger, O. Schmidt, M. Sebastian, L. Nyulaszi, and E. Niecke, Chem. Eur. J. 2002, 8, |
|
|
2188. |
|
110. |
(a) E. O. Fischer and R. Restmeier, Z. Naturforsch. 1983, 38b, 582. (b) F. R. Kreissl, T. |
|
|
Lehotkay, C. Ogric, and E. Herdtweck, Organometallics 1997, 16, 1875. (c) S. Dovesi, |
|
|
E. Solari, R. Scopelliti, and C. Floriani, Angew. Chem. Int. Ed. Engl. 1999, 38, |
|
|
2388. |
|
111. |
(a) W. W. Schoeller, D. Eisner, S. Grigoleit, A. J. B. Rozhenko, and A. Alijah, J. Am. |
|
|
Chem. Soc. 2000, 122, 10115. (b) W. W. Schoeller, A. J. B. Rozhenko, and A. Alijah, |
|
|
J. Organomet. Chem. 2001, 617, 435. |
|
372STABLE SINGLET CARBENES
112.E. Despagnet, K. Miqueu, H. Gornitzka, P. W. Dyer, D. Bourissou, and G. Bertrand,
J. Am. Chem. Soc. 2002, 124, 11834.
113.N. Merceron, K. Miqueu, A. Baceiredo, and G. Bertrand, unpublished results.
114.J. C. Green, R. G. Scurr, P. L. Arnold, and F. G. N. Cloke, Chem. Commun. 1997, 1963.
¨ |
and P. W. Roesky, J. Organomet. |
115. W. A. Herrmann, K. Ofele, M. Elison, F. E. Kuhn,¨ |
Chem. 1994, 480, C7. N. Kuhn, T. Kratz, D. Bla¨ser, and R. Boese, Inorg. Chim. Acta 1995, 238, 179.
116. (a) M. H. Voges, C. Romming, and M. Tilset, Organometallics 1999, 18, 529. (b) C. D. Abernethy, J. A. C. Clyburne, A. H. Cowley, and R. A. Jones, J. Am. Chem. Soc. 1999, 121, 2329.
117. (a) W. A. Herrmann, M. Elison, J. Fischer, C. Kocher, and G. R. J. Artus, Angew. Chem. Int. Ed. Engl. 1995, 34, 2371. (b) C. L. Yang, H. M. Lee, and S. P. Nolan, Org. Lett. 2001, 3, 1511. (c) M. V. Bakler, B. W. Skelton, A. H. White, and C. C. Williams, J. Chem. Soc., Dalton Trans. 2001, 111.
118. (a) A. Furstner and A. Leitner, Synthesis 2001, 2, 290. (b) C. W. K. Gstottmayr, V. P. W. Bohm, E. Herdweck, M. Grosche, and W. A. Herrmann, Angew. Chem. Int. Ed. Engl. 2002, 41, 1363.
119. (a) S. Caddick, F. G. N. Cloke, G. K. B. Clentsmith, P. B. Hitchcock, D. McKerrecher,
L.R. Titcomb, and M. R. V. Williams, J. Organomet. Chem. 2001, 617, 635. (b) C. L. Yang and S. P. Nolan, Organometallics, 2002, 21, 1020.
120.(a) W. P. Bohm, C. W. K. Gstottmayr, T. Weskamp, and W. A. Herrmann, Angew. Chem. Int. Ed. Engl. 2001, 40, 3387. (b) W. P. Bohm, T. Weskamp, C. W. K. Gstottmayr, and
W.A. Herrmann, Angew. Chem. Int. Ed. Engl. 2000, 39, 1602.
121.G. A. Grasa and S. P. Nolan, Org. Lett. 2001, 3, 119.
122.(a) S. R. Stauffer, S. W. Lee, J. P. Stambuli, S. I. Hauck, and J. F. Hartwig, Org. Lett. 2000, 2, 1423. (b) S. Lee and J. F. Hartwig, J. Org. Chem. 2001, 66, 3402.
123.I. E. Marko, S. Sterin, O. Buisine, R. Mignani, P. Branlard, B. Tinant, and J. P. Declercq,
Science 2002, 298, 204.
124.A. Fu¨rstner, Angew. Chem. Int. Ed. Engl. 2000, 39, 3013.
125.F. W. Michelotti and W. P. Keaveney, J. Polym. Sci. 1965, A3, 895.
126.S. T. Nguyen, L. K. Johnson, R. H. Grubbs, and J. W. Ziller, J. Am. Chem. Soc. 1992, 114, 3974.
127.(a) M. Scholl, T. M. Trnka, J. P. Morgan, and R. H. Grubbs, Tetrahedron Lett. 1999, 40, 2247. (b) T. Weskamp, F. J. Kohl, W. Hieringer, D. Gleich, and W. A. Herrmann, Angew. Chem. Int. Ed. Engl. 1999, 38, 2416. (c) L. Ackermann, A. Fu¨rstner, T. Weskamp, F. J. Kohl, and W. A. Herrmann, Tetrahedron Lett. 1999, 40, 4787. (d) J. Huang, E. D. Stevens, S. P. Nolan, and J. L. Petersen, J. Am. Chem. Soc. 1999, 121, 2674.
128.M. Scholl, S. Ding, C. W. Lee, and R. H. Grubbs, Org. Lett. 1999, 1, 953.
129.R. R. Schrock, Tetrahedron 1999, 55, 8141.
130.D. Enders and H. Gielen, J. Organomet. Chem. 2001, 617, 70.
131.M. T. Powell, D. R. Hou, M. C. Perry, X. H. Cui, and K. Burgess, J. Am. Chem. Soc. 2001, 123, 8878.
132.T. J. Seiders, D. W. Ward, and R. H. Grubbs, Org. Lett. 2001, 3, 3225.
133.(a) D. Enders and U. Kalfass, Angew. Chem. Int. Ed. Engl. 2002, 41, 1743. (b) M. S. Kerr,
J.Read de Alaniz, and T. Rovis, J. Am. Chem. Soc. 2002, 124, 10298.
REFERENCES 373
134.A. C. Hillier, W. J. Sommer, B. S. Yong, J. L. Petersen, L. Cavallo, and S. P. Nolan,
Organometallics, 2003, in press.
135.T. Weskamp, F. J. Kohl, W. Hieringer, D. Gleich, and W. A. Herrmann, Angew. Chem. Int. Ed. Engl. 1999, 38, 2416.
136.A. Fu¨rstner, L. Ackermann, B. Gabor, R. Goddard, C. W. Lehmann, R. Mynnot,
F.Stelzer, and O. R. Thiel, Chem. Eur. J. 2001, 7, 3236.
137.(a) J. Schwartz, V. P. W. Bohm, M. G. Gardiner, M. Grosche, W. A. Herrmann, W. Hieringer, and G. Raudaschl-Sieber, Chem. Eur. J. 2000, 1773. (b) J. S. Kingsbury, S. B. Garber, J. M. Giftos, B. L. Gray, M. M. Okamoto, R. A. Farrer, J. T. Fourkas, and A. H. Hoveyda, Angew. Chem. Int. Ed. Engl. 2001, 40, 4251. (c) M. Ahmed, T. Arnauld,
A.G. M. Barrett, D. C. Braddock, and P. A. Procopiou, Synlett 2000, 1007.
(d) S. C. Schurer, S. Gessler, N. Buschmann, and S. Blechert, Angew. Chem. Int. Ed. Engl. 2000, 39, 3898. (e) M. Mayr, B. Mayr, and M. R. Buchmeiser, Angew. Chem. Int. Ed. Engl. 2001, 40, 3839.
138.(a) P. S. Skell and A. Y. Garner, J. Am. Chem. Soc. 1956, 78, 3409 and 5430. (b) P. S. Skell and R. C. Woodworth, J. Am. Chem. Soc. 1956, 78, 4496 and 6427.
