
clavien_atlas_of_upper_gastrointestinal_and_hepato-pancreato-biliary_surgery2007-10-01_3540200045_springer
.pdf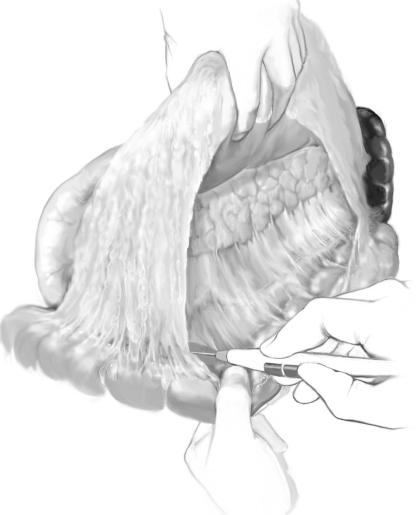
Pancreatic Enucleation |
821 |
|
|
|
Procedures |
|
Open Enucleation |
|
Geoffrey B. Thompson |
|
|
STEP 1 |
Setup, transverse epigastric incision, exploration, entry into lesser sac |
|
Plasma glucose concentrations should be checked every 20–30min with the patient |
|
|
|
off all glucose-containing fluids (insulinoma patients only). |
|
The patient is best positioned supine with arms tucked at sides. |
|
A transverse epigastric incision is best. A third-arm mechanical retractor facilitates |
|
the exposure and allows a thorough abdominal/pelvic exploration to be carried out. |
|
First, the gastrocolic omentum is mobilized off the transverse colon from left to right, |
|
entering the lesser sac; the stomach and the omentum are held cephalad by the second |
|
assistant, and the transverse colon is retracted caudad, exposing the pancreas. |
822 |
SECTION 6 |
Pancreas |
|
|
|
STEP 2 |
Kocherization, mobilization body and tail, inspection, and palpation |
|
|
|
|
Before further mobilization, the pancreas is inspected and palpated carefully. If the tumor is readily apparent on the surface or edge of gland and its location is consistent with preoperative localization studies, further mobilization may be limited to the involved portion of the pancreas, unless there is a familial syndrome or other concerns predisposing the patient to multiple neoplasms.
For tumors in the head and uncinate region, the duodenum is widely Kocherized out to and including the ligament of Treitz; division of the right gastroepiploic vessels on the anterior surface of the pancreatic head and the anterior inferior pancreatoduodenal vein to the uncinate facilitates exposure of the head and uncinate and reduces the risk of inadvertent vascular injury and bleeding during further dissection; transpancreatic palpation of the head and uncinate allows localization of tumors in this region.
Pancreatic Enucleation |
823 |
|
|
STEP 3
When the tumor is in the neck of the pancreas, a plane is developed between the underside of the neck of the gland and the underlying portal vein – the superior mesenteric vein junction. After exposing the superior mesenteric vein at the inferior edge of the neck of the pancreas, gentle blunt dissection with an index finger or a small cherrytipped sucker completes the dissection under direct vision; care is taken to stay directly on top of the vein throughout the dissection to avoid injury to lateral venous tributaries to the uncinate. In patients with insulinoma, this is usually an easy dissection.
824 |
SECTION 6 |
Pancreas |
|
|
|
STEP 4
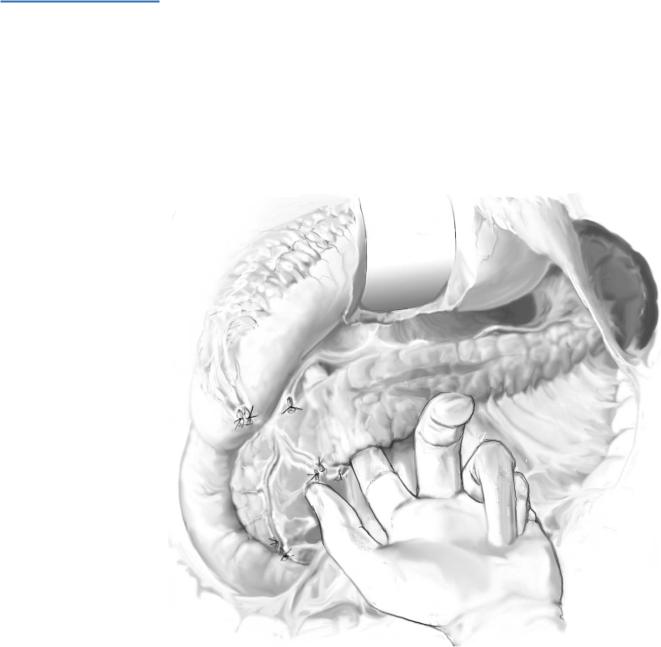
When the tumor is in the body of the pancreas, dissection continues along the avascular inferior border of the body of the gland. After incising this plane, the body of the pancreas is mobilized from the inferior to superior border (A-1).
When the tumor is in the tail of the pancreas, mobilization of the spleen by incising its lateral peritoneal attachments to the kidney and diaphragm usually allows best exposure. Short gastric vessels are not divided until the decision is made regarding splenic-preservation (A-2).
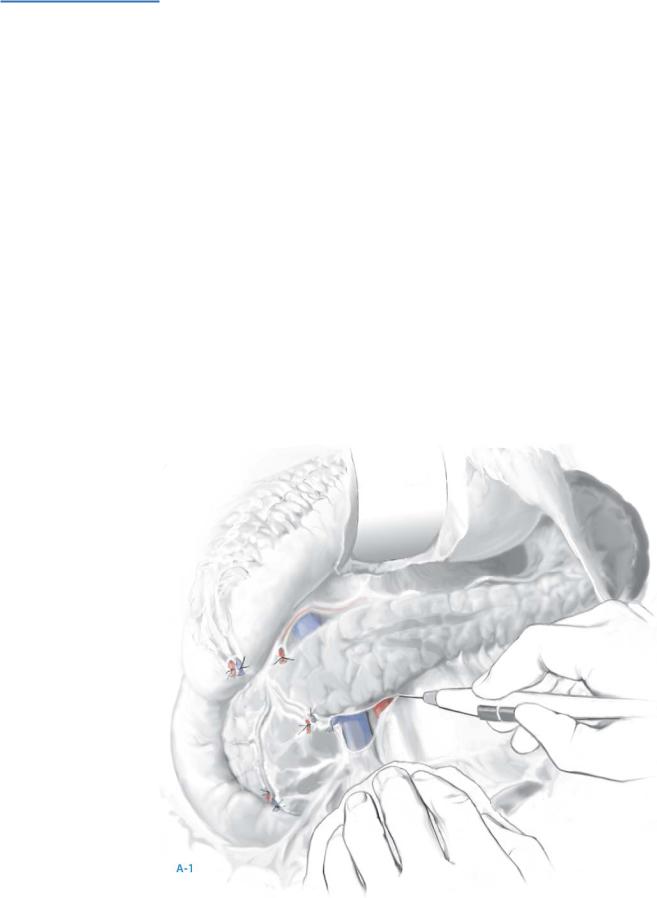
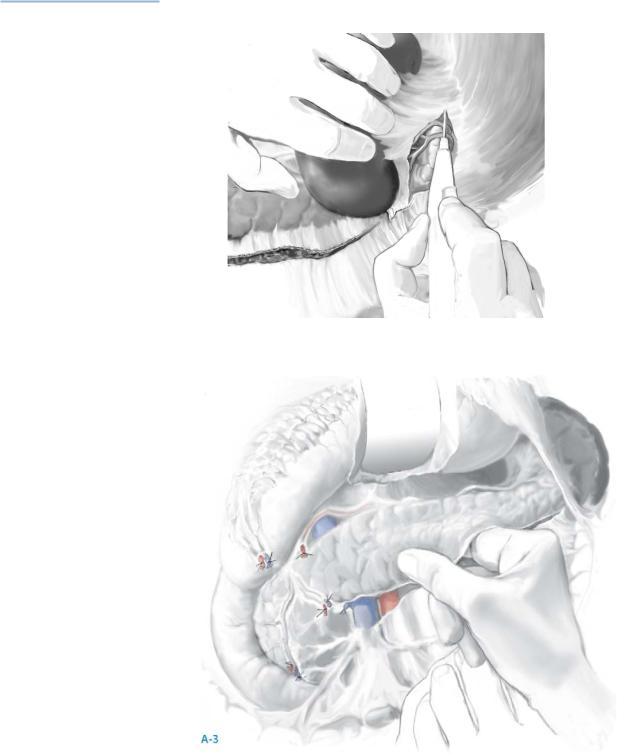
When the tumor is occult, all these maneuvers should be performed prior to intraoperative ultrasonography (IOUS); bimanual and bidigital palpation allow the gland to be examined from head to tail; suspicious lymph nodes are excised for frozen section analysis (A-3).
Real-time IOUS is next performed using a 6-mHz transducer in the longitudinal and transverse axes. Islet cell neoplasms appear as hypoechoic masses within the pancreatic parenchyma, are more firm in texture than the surrounding parenchyma, and are tan to reddish-brown.
Islet cell neoplasms typically have a rim of vascular enhancement not seen with lymph nodes on color-flow Doppler examination.
Further palpation of lesions on ultrasonography often reveals subtle thickening within the pancreas not appreciated during the initial exploration.
If doubt of the findings exists, ultrasonography-guided fine-needle aspiration provides immediate cytologic evaluation; this is especially important for occult islet cell neoplasms deep within the head of gland abutting the pancreatic and/or bile ducts; this situation requires pancreatoduodenectomy, and absolute cytologic confirmation of an islet cell neoplasms is essential.
Pancreatic Enucleation |
825 |
|
|
STEP 4 (continued)
A-2
826 |
SECTION 6 |
Pancreas |
|
|
|
STEP 5 |
Enucleation |
|
|
Once the site of the tumor is ascertained and no other abnormalities are noted, a |
|
|
|
|
|
decision must be made regarding enucleation versus resection; over two-thirds of |
|
|
insulinomas can be enucleated safely with attention to detail. |
|
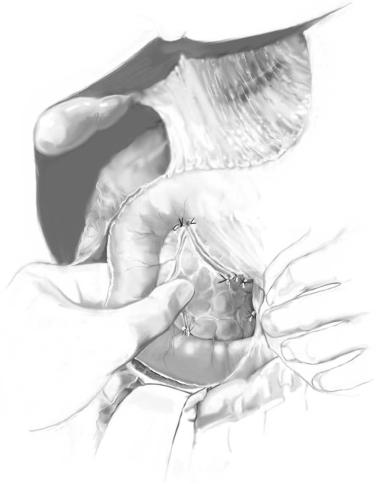
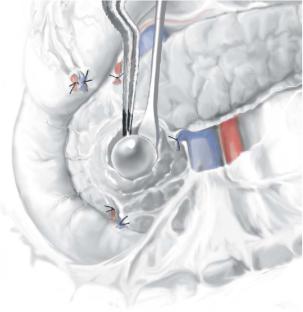
For palpable tumors on the edge of the gland, anterior surface, or posterior surface, the very thin operculum of pancreatic tissue overlying the tumor is carefully incised using a combination of bipolar cautery and a fine-tipped hemostat, the underlying insulinoma exposed, and a traction suture is placed deep into the tumor in figure-of- eight fashion to avoid fracture. Constant gentle elevation of the traction suture allows direct visualization of the small feeding vascular tributaries, which can be managed with bipolar cautery but infrequently require fine metallic clips. The pancreatic parenchyma is dissected away from the tumor using a fine endarterectomy spatula. Near the base of the enucleation, the endarterectomy spatula really helps to push the parenchyma away from the adenoma, especially when enucleating tumors are abutting the pancreatic duct.
Once enucleation is complete, ultrasonography confirms the integrity of the main pancreatic duct. In the past, secretin was administered to dilate the pancreatic duct to demonstrate major leaks from the enucleation site.
Pancreatic Enucleation |
827 |
|
|
STEP 5 (continued) |
Enucleation |
|
When the spleen is sizable or the patient is thin, the splenic artery and vein can be |
|
|
|
freed from the pancreas by individually dividing venous and arterial tributaries with |
|
fine metal clips and/or a harmonic scalpel; concern regarding patency of the splenic |
|
vein should lead to splenectomy or arterial and venous ligation, leaving the spleen based |
|
on short gastric vessels; the former is essential for a larger spleen. This avoids future |
|
development of left-sided portal hypertension and gastric varices. |
|
All malignant islet cell neoplasms require oncologic principles with distal pancreatec- |
|
tomy and splenectomy or pancreatoduodenectomy including regional lymphadenec- |
|
tomy. |
|
After enucleation/resection, the abdomen is irrigated and hemostasis assured. |
|
The stomach and greater omentum are draped over the colon and the abdomen |
|
closed. |
|
Major ductal disruption in the body and tail of the gland is best managed with imme- |
|
diate distal pancreatectomy. |
|
A major ductal injury in the head of the pancreas is managed by (1) suture closure |
|
of the duct with fine absorbable suture and drainage, (2) closure of the hole over a tiny |
|
silastic stent passed across the papilla with placement of the Roux limb over the |
|
enucleation site, or (3) pancreatoduodenectomy (<2% of patients). External drainage |
|
is strongly suggested in all patients. |
|
Once the insulinoma is removed, plasma glucose concentrations are drawn every |
|
15min; increases in serum glucose concentration by 20mg/dl in the first 30min after |
|
excision usually indicate a cure, but false positive and false negative studies are possible; |
|
in some centers, rapid insulin assays are available. |
|
The enucleation site is left open and drains are placed nearby. |
|
Tumors larger than 2cm centralized within the body or tail of the pancreas are prob- |
|
ably best managed by distal pancreatic resection with or without splenectomy unless |
|
they are located excentrically . |
|
For tumors in the tail of the gland, it is usually easy to separate the splenic artery and |
|
vein from the gland, and the pancreatic tail is removed using a stapling device; the staple |
|
line is reinforced with a row of absorbable horizontal mattress sutures that incorporate |
|
the pancreatic duct. Drains are placed. |
|
For larger tumors in the body of the pancreas, splenic preservation can be accom- |
|
plished in two ways. In obese patients, the splenic artery and vein are divided 1–1.5cm |
|
outside the splenic hilum, leaving the spleen based on the short gastric vessels provided |
|
the spleen is not enlarged. Once the spleen and blood supply are separated from the |
|
pancreas, the splenic artery is re-divided and ligated at its origin. The splenic vein is also |
|
re-divided and ligated at its junction with the superior mesenteric/portal vein junction, |
|
and the body and tail are removed with a stapler. |
828 |
SECTION 6 |
Pancreas |
|
|
|
|
Laparoscopic Enucleation |
|
|
Michel Gagner |
|
|
|
|
STEP 1 |
Operative room positioning/trocar placement |
|
|
The patient is placed under general anesthesia with orotracheal intubation. |
|
|
|
A nasogastric tube and Foley urinary catheter are put in place. Prophylactic parenteral antibiotics are given.
The patient is positioned in the lithotomy position with a wedge under the left flank (45° of elevation).
The surgeon stands between the patient’s legs, the first assistant to patient’s right, and the scrub nurse to the patient’s left.
Two monitors are positioned at each of the patient’s shoulders.
A five-trocar technique is used (10-mm trocar at umbilicus for 10mm, 30° laparoscope); the size and position of the other four trocars vary with patient habitus, but a 12-mm trocar in the left mid-axillary line is used to introduce the linear stapler.
Pancreatic Enucleation |
829 |
|
|
STEP 2 |
Pancreatic exposure and mobilization |
|
Exploratory laparoscopy is performed first to exclude local or distant extension of the |
|
|
|
neoplasm. |
|
The small bowel is removed from the operative field and the table rotated/inclined |
|
with the left side up and in reverse Trendelenberg to obtain the best exposure. |
|
The lesser sac is exposed by opening the gastrocolic ligament widely inferior to the |
|
gastroepiploic arcade using an ultrasonic scalpel (Ultracision, United States Surgical Co., |
|
CT) or electrothermal bipolar vessel sealing device (LigaSure Lap, Tyco Healthcare, CO). |
|
The splenic flexure of the colon is mobilized inferiorly to expose the pancreatic tail; if |
|
further exposure is needed, the short gastric vessels are transected. |
|
The retroperitoneum is incised along the inferior and superior border of the |
|
body/tail of pancreas; the mesenteric vessels are identified at the uncinate process, as |
|
well as the splenic artery at the superior border of the pancreas. |
830 |
SECTION 6 |
Pancreas |
|
|
|
STEP 3 |
Ultrasonography |
|
|
|
|
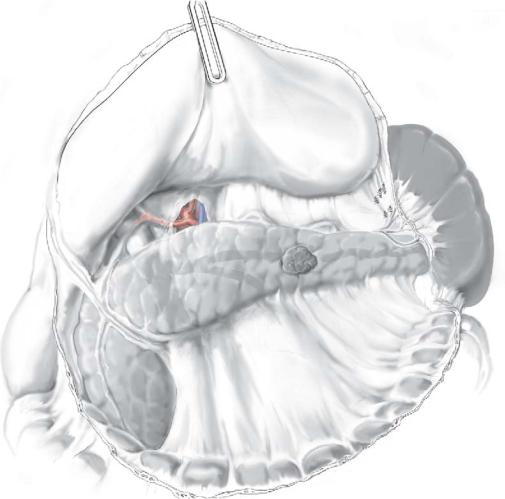
Endoscopic ultrasonography is performed routinely; the aim is to localize the tumor, define resection margin, exclude secondary lesion (both hepatic or pancreatic), and define the relationship with the main vessels and pancreatic duct.
The probe we use fits through a 10-mm trocar; its tip can be angulated for maximal surface contact. For small lesions, a water balloon is placed between the probe and the pancreatic body to improve resolution.
The ideal treatment is enucleation; however, with a larger tumor or one deep or localized too close to the main vessels or pancreatic duct, a pancreatic tail resection, with or without splenectomy, is preferred.
STEP 4 |
Enucleation of pancreatic tumor |
|
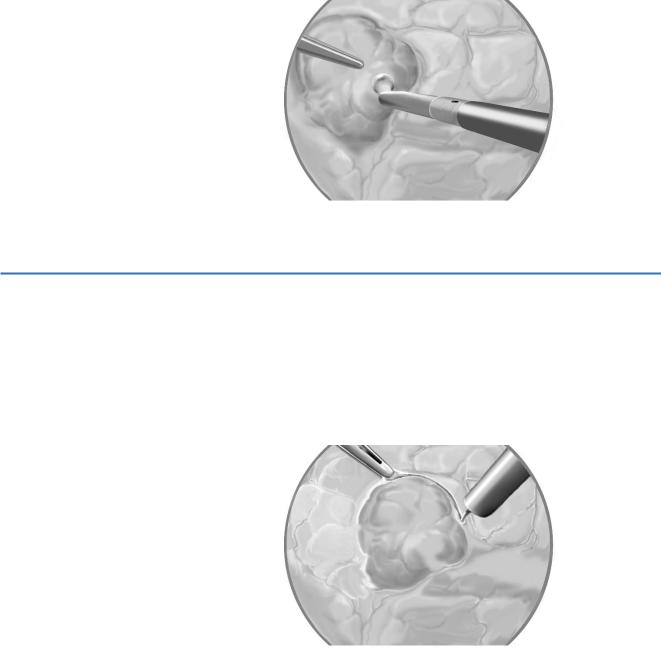
A pancreatotomy is performed in a circle a few millimeters from the edge of the tumor. |
|
|
|
An ultrasonic scalpel is used to dissect the tumor from the normal parenchyma. |
|
As the plane of dissection goes deeper, small vessels are cauterized or ligated with |
|
ultrasonic energy. |
|
An irrigation-suction cannula keeps the field free of blood; if hemorrhage occurs, |
|
pressure is applied for several minutes. |
