clavien_atlas_of_upper_gastrointestinal_and_hepato-pancreato-biliary_surgery2007-10-01_3540200045_springer
.pdf
Resection for Neoplasms of the Pancreas |
791 |
|
|
Distal Pancreatectomy
Eric Nakakura, Mark Duncan, Frederick Eckhauser
Introduction
Unlike cancer of the head of the pancreas, ductal adenocarcinoma of the pancreas is less often resectable because of the delay in symptoms and diagnosis. However, when feasible, the survival after R0 resection of a distal ductal cancer is similar to that of proximal resections. With the improvements in imaging as well as screening of more high-risk populations, the number of distal pancreatectomies for malignancy appears to be increasing. Moreover, cystic neoplasms of the pancreas and non-functional neuroendocrine neoplasms also occur in the body/tail of the pancreas and are more commonly “resectable.”
Indications and Contraindications
Indications |
■ |
Ductal adenocarcinoma of the body and tail of pancreas |
|
|
Major visceral vessel encasement (celiac axis, proximal splenic or hepatic artery), |
Contraindications |
■ |
|
|
|
widespread local metastatic disease, or distant metastases [liver, peritoneum, distal |
|
|
nodes (celiac, superior, mesenteric or para-aortic)] |
|
■ |
Local extension to spleen, stomach, colon, or left kidney is not always a contra- |
|
|
indication |
Preoperative Investigation and Preparation for the Procedure
History: |
Weight loss, abdominal or back pain, pancreatitis, new onset |
|
diabetes mellitus, family history of pancreatic cancer |
Clinical evaluation: |
Jaundice, nutritional status, ascites, palpable left supraclavicular |
|
node, signs of sinistral portal hypertension (splenomegaly, |
|
gastric varices) |
Laboratory tests: |
Tumor markers (i.e., CA19–9, CEA), liver function tests |
ERCP, EUS, CT: |
Assessment of resectability (no distant metastases, no major |
|
visceral vessel encasement), staging extent of tumor |
Other considerations: Preoperative vaccination against pneumococcus, meningococcus, and Haemophilus influenzae; staging laparoscopy (before vs at time of proposed resection)
792 SECTION 6 Pancreas
Procedure: Distal Pancreatectomy
Access: An upper midline or left subcostal incision is best, depending on the angle of the costal margin.
STEP 1
The exposure and exploration are aided markedly by installation of a fixed blade retractor.
A careful, systematic exploration of the abdomen is performed to detect distant metastases (peritoneum, liver, distant nodal basins).
The lesser sac is entered through the gastrocolic ligament to expose the entire ventral surface of the pancreas from the gastroduodenal artery medially to the splenic hilum laterally.
The splenic flexure of the colon is mobilized inferiorly; cephalad retraction of the stomach and caudad retraction of transverse colon exposes the pancreatic bed.
Vessels along the greater curvature of the stomach, including the vasa brevia, are ligated with fine silk ties and divided.
Resection for Neoplasms of the Pancreas |
793 |
|
|
|
|
STEP 2 |
Deliver the pancreas and spleen from the retroperitoneum |
|
|
(see also chapter “Distal Pancreatectomy”) |
|
|
|
|
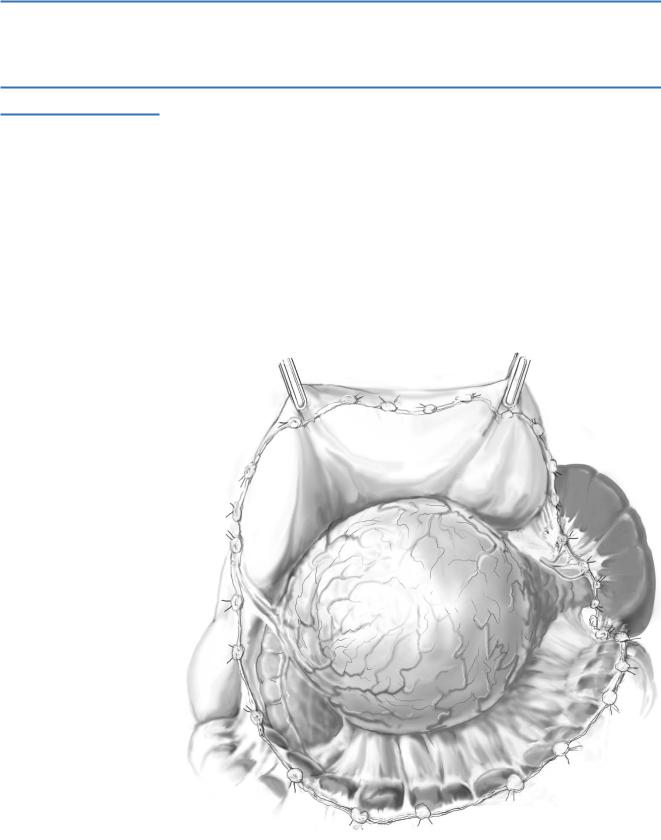
The peritoneum along the inferior border of the pancreas is incised sharply or with electrocautery, maintaining a margin between the edge of the tumor mass.
The spleen is then mobilized laterally and posteriorly by dividing its peritoneal attachments.
The splenocolic ligament is next divided carefully to prevent injuring the splenic flexure of the colon. A thorough bimanual palpation of the pancreas is performed to assess mobility of the mass and the tumor stage.
If the tumor is adherent to the stomach, diaphragm, mesocolon/colon, retroperitoneum, adrenal gland or kidney, an en bloc resection is advised.
The spleen and pancreas are mobilized anteromedially from the retroperitoneum using either blunt or sharp dissection depending on tumor extension.
794 |
SECTION 6 |
Pancreas |
|
|
|
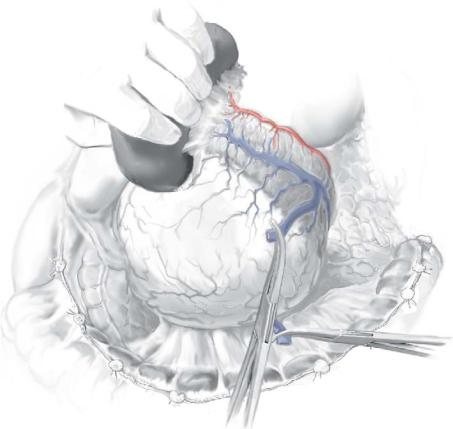
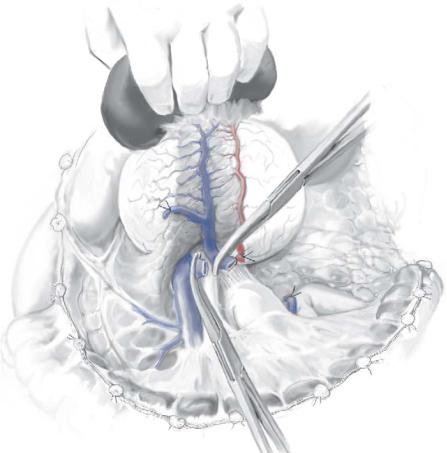
STEP 3 |
Isolate and secure the splenic vasculature |
|
|
|
|
The splenic artery is identified and suture ligated as it passes along the posterosuperior border of the body and tail of the pancreas.
The splenic vein is generally identified inferior and posterior to the splenic artery, where it is ligated and divided; if the inferior mesenteric vein joins the splenic vein near the confluence of the splenic and superior mesenteric veins, it may be preserved – otherwise, it can be ligated with impunity.
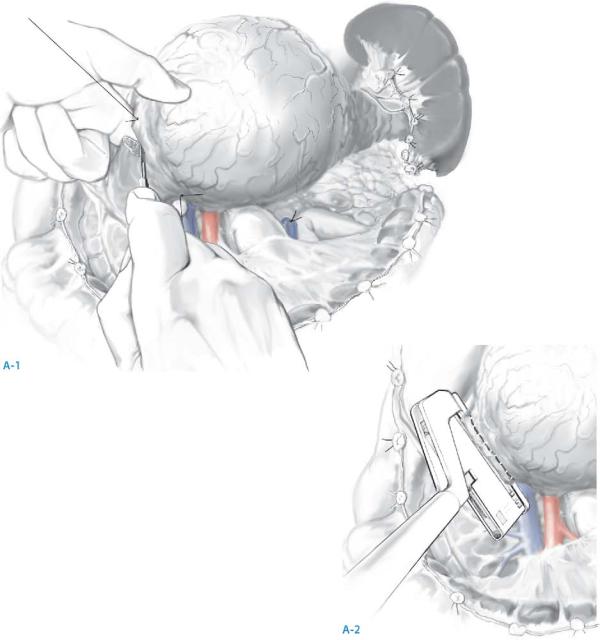
Resection for Neoplasms of the Pancreas |
795 |
|
|
|
|
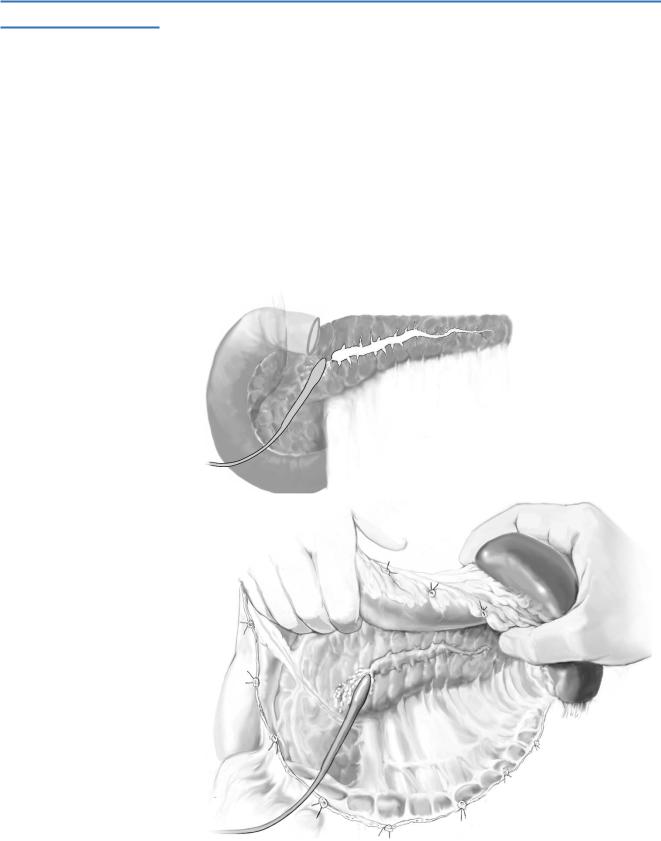
STEP 4 |
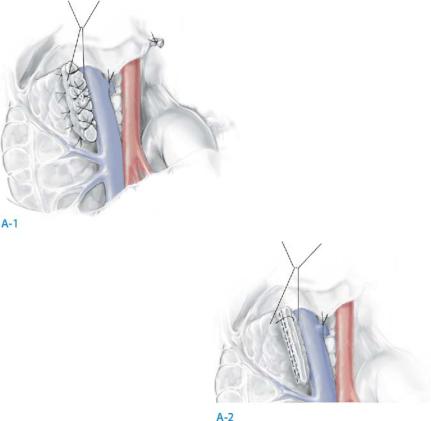
Divide the pancreas (A-1, A-2) |
|
|
Stay sutures are placed through the superior and inferior margins of the pancreas on |
|
|
|
|
|
both sides of the transection margin to provide hemostasis and traction. |
|
The pancreas is divided sharply to minimize the effects of cautery artifact on the frozen section of the proximal margin, which is imperative to check for tumor involvement.
Alternatively, the pancreas can be divided using a linear stapler at the margin of the pancreatic remnant provided the pancreas is not too thick (≥1cm).
The resected specimen of the distal pancreas and spleen is sent for frozen section evaluation of the proximal pancreatic margin.
796 |
SECTION 6 |
Pancreas |
|
|
|
STEP 5 |
Close the pancreatic duct |
|
|
|
|
The transected pancreatic duct is closed specifically with a fine, polypropylene suture placed either as a figure-of-eight or as a U stitch proximal to the divided open end of the duct; when the pancreas is divided with a stapler technique, suture closure of pancreatic duct is not necessary (A-1).
The divided end of the pancreatic remnant is closed with interrupted, partially overlapping 3-0 polypropylene mattress sutures.
When a stapling instrument used, it is not necessary to oversew the divided end of pancreas; some surgeons, however, prefer a continuous suture of fine polypropylene to reinforce the staple suture. If the pancreas is thick, however, a staple closure is not suggested (A-2).
A closed suction drain is placed in the bed of the resected gland in proximity to but not in direct apposition with the divided end of the pancreas.
Resection for Neoplasms of the Pancreas |
797 |
|
|
Postoperative Care and Tests
Postoperative surveillance in an intermediate care or general care unit; intensive care unit monitoring should not be necessary
Monitor hemoglobin, coagulation parameters, and acid/base and electrolyte balance at regular intervals
Postoperative Complications
■Short term:
–Intra-abdominal bleeding
–Pancreatic fistula
–Peripancreatic fluid collection
–Subphrenic abscess
–Pleural effusion
–Diabetes mellitus
–Portal vein thrombosis
–Ascites (differentiate pancreatic from non-pancreatic origin)
■Long term:
–Pancreatic exocrine insufficiency
–Diabetes mellitus
–Local or distant recurrence
–Pancreatic pseudocyst

798 |
SECTION 6 |
Pancreas |
|
|
|
|
Tricks of the Senior Surgeon |
|
■The position of the patient is important; a moderate reverse Trendelenberg position with the patient rolled slightly toward the right facilitates the exposure by dropping the pancreas and spleen away from the left hemidiaphragm and by dropping the left transverse and splenic flexure of the colon away from the operative field.
■When easily accessible, the proximal splenic artery should be secured prior to completely mobilizing the pancreas and spleen from the retroperitoneum; this enables a rapid, secure, immediate hemostasis if the splenic capsule is torn during mobilization.
■The splenic artery and vein are suture ligated and divided individually rather than securing both together with a mass ligature; this approach decreases the risk of postoperative splenic arteriovenous fistula.
■Whenever possible, monofilament suture material rather than silk should be used to close the divided end of the pancreas. Monofilament, such as polypropylene, has a lower slip coefficient and can be pulled more easily (and potentially with less trauma) through tissue; monofilament lacks interstices and may be more resistant to infection than polyfilamentous sutures.
Enteric Drainage of Pancreatic Fistulas with Onlay Roux-en-Y Limb
Peter Shamamian, Stuart Marcus
Introduction
The majority of pancreatic fistulae will resolve with conservative measures, including improving pancreatic duct drainage through the ampulla, nutritional support, and somatostatin analogues. When pancreatic fistulae result from pancreatic ductal disruption and pancreatic secretions produced in a portion of the pancreas do not flow into the GI tract, operative intervention is indicated.
Indications and Contraindications
Indications in |
■ |
Pancreatic ductal disruption secondary to pancreatic trauma |
Non-Resolving Fistulae |
■ |
Fistulae following debridement of pancreatic necrosis |
|
■ |
Fistulae from complications of pancreatic surgery |
|
|
(i.e., enucleation of endocrine neoplasms) |
|
■ |
Fistulae from pancreatic injury during surgery on juxtapancreatic organs |
|
|
(i.e., stomach, colon, left kidney, left adrenal, spleen) |
|
■ |
Fistulae secondary to external drainage of pancreatic pseudocysts |
|
■ |
Fistulae associated with pancreatic ascites |
|
■ |
Pancreato-pleural fistulae |
|
|
Ongoing acute pancreatic inflammation |
Contraindications |
■ |
|
|
■ |
Pancreatic abscess |
|
■ |
Undrained peri-pancreatic fluid collection |
Preoperative Investigation and Preparation for the Procedure
History: |
Pancreatitis, pancreatic trauma, pancreatic pseudocyst, prior |
|
pancreatic surgery, prior non-operative therapy with internal or |
|
external drains; exclude alcohol abuse, cirrhosis, portal hypertension |
Clinical evaluation: |
Quantify fistula output, optimize nutritional status, provide adequate |
|
external drainage, protect the skin from pancreatic secretions |
Laboratory tests: |
Serum electrolytes, amylase, lipase, and liver chemistries, bacterial |
|
culture, cytology and amylase of any drained peritoneal or pleural |
|
fluid |
Preoperative Imaging |
■ |
CT: exclude presence of undrained collections or abscess |
|
■ |
ERCP: delineate pancreatic ductal anatomy, site of duct disruption, obstructing |
|
|
strictures or calculi |
|
■ |
Fistulogram: anatomy of pancreatic segment from which fistulous tract originates; |
|
|
adequate external preoperative drainage is essential. |
|
■ |
Angiogram: if suspicion of a pseudoaneurysm |