
clavien_atlas_of_upper_gastrointestinal_and_hepato-pancreato-biliary_surgery2007-10-01_3540200045_springer
.pdf

762 |
SECTION 6 |
Pancreas |
|
|
|
|
|
|
Postoperative Tests |
|
|
|
■ |
First 24h, monitor for hemorrhage |
|
|
■ |
Monitor serum glucose concentrations for possible glucose intolerance even in |
|
|
|
patients who had not required insulin preoperatively |
|
|
■ |
Reinforce the need for a progressive reduction in narcotic analgesic (this continues |
|
|
|
after discharge from hospital) |
|
|
■ |
Although the final outcome is determined only when the patient is free of narcotic |
|
|
|
use, pain scales may be of some value |
|
Local Postoperative Complications
■Short term:
–Intra-abdominal hemorrhage
–Pancreatic ductal leak (persistent drain output)
–Glucose intolerance resulting from the stress of operation
–Alcohol withdrawal syndrome
–Wound infection
■Long term:
–Failure to resolve pain
–Ongoing narcotic addition
–Recurrent episodes of pancreatitis
–Progressive loss of pancreatic function
Tricks of the Senior Surgeon
■It may be difficult to palpate the duct, and various maneuvers may help.
■Kocherize the duodenum and palpate carefully the head of the pancreas.
■Perform intraoperative ultrasonography.
■Choose an area in the mid body of the pancreas and perform a vertical incision in hope of bisecting the duct.
■Once some incision has been made in the parenchyma, the surgeon may safely massage the tail and proximal body of the pancreas in hope of expressing pancreatic juice.
■Avoid trying to access the pancreatic duct directly over the superior mesenteric vein/portal vein/splenic vein junction – too deep an incision may lead to serious bleeding.
■Concentrate on getting a good size match between the ductal incision and the jejunal incision; mismatches in size can lead to pancreatic leak.

Resection for Neoplasms of the Pancreas
Tina W.F. Yen, Douglas B. Evans (Proximal Resection),
Sergio Pedrazzoli, Claudio Pasquali, Cosimo Sperti (Central Resection),
Eric Nakakura, Mark Duncan, Frederick Eckhauser (Distal Pancreatectomy)
Introduction
With the increased experience of centers of expertise in pancreatic diseases, pancreatectomy for neoplasms has become routine, with acceptable morbidity and mortality. Improvements in imaging modalities and heightened awareness of less well-appreciated pancreatic neoplasms (non-functional neuroendocrine neoplasms, the spectrum of primary cystic neoplasms of the pancreas, and especially intraductal papillary mucinous neoplasms) has increased the visibility of formal pancreatectomy. The following sections address specific types of anatomic pancreatic resections.
Proximal Resection
Tina W.F. Yen, Douglas B. Evans
Introduction
Kausch was the first to describe a pancreatoduodenectomy, but Whipple, who described pancreaticoduodenectomy for pancreatic head adenocarcinoma in 1935 as a two-stage operation, has largely been given credit by the eponym. Waugh and Clagett modified the operation in 1946 to its current one-stage procedure. The operation involves the en bloc removal of the pancreatic head, duodenum, gallbladder, and bile duct, with the gastric antrum. Traverso and Longmire popularized the pylorus-preserving technique. The goal of the operation is complete removal of the neoplasm (R0 resection). There are no data to support debulking (R2 resection) in patients with adenocarcinoma of the pancreas. Therefore, the preoperative assessment of local tumor resectability is of critical importance.
Indications and Contraindications
General Principles |
■ |
We advocate objective, anatomic criteria for defining resectability based on high |
|
|
quality CT images. |
|
■ |
Local tumor resectability [relationship of the neoplasm to the celiac axis, superior |
|
|
mesenteric artery (SMA) and superior mesenteric-portal vein (SMPV) confluence] |
|
|
cannot be determined accurately at laparotomy before gastric and pancreatic |
|
|
transection; thus, preoperative assessment of critical tumor-vessel relationships |
|
|
is mandatory. |

764 |
SECTION 6 |
Pancreas |
|
|
|
|
|
|
|
Resectable neoplasms have the following CT characteristics: |
|
Indications |
■ |
|
|
|
|
– Normal fat plane between the low-density tumor and the superior mesenteric |
|
|
|
artery and superior mesenteric vein (SMV) |
|
|
|
– Absence of extrapancreatic disease |
|
|
|
– Patent SMPV confluence (assumes ability of the surgeon to resect and reconstruct |
|
|
|
isolated segments of the SMV or SMPV) |
|
|
|
– No direct tumor extension to the celiac axis or SMA |
|
|
■ |
“Borderline” resectable neoplasms include: |
|
|
|
– Short segment occlusion of the SMPV confluence with an adequate vessel for |
|
|
|
grafting above and below the site of occlusion (assumes the technical ability to |
|
resect and reconstruct the SMV or SMPV)
– Neoplasms which demonstrate short-segment (usually <1cm) abutment of the common or proper hepatic artery or the SMA on high-quality CT
Absolute Contraindications ■ Extrapancreatic metastatic disease
■Neoplasms encasing the celiac axis or SMA (anything more than short-segment abutment)
Preoperative Investigations and Preparation for the Procedure
Predisposing factors: |
Tobacco, obesity, impaired glucose tolerance, smoking, |
|
family history (~10%), associated syndromes [familial |
|
pancreatitis, Peutz-Jeghers syndrome, familial atypical |
|
multiple-mole melanoma (FAMMM) syndrome, hereditary |
|
nonpolyposis colon cancer (HNPCC)] |
Physical examination: |
Jaundice, left supraclavicular adenopathy, ascites, nutri- |
|
tional status (weight loss), cardiovascular health, perform- |
|
ance status (most important for assessment of operativel |
|
risk) |
Laboratory tests: |
CA 19–9, CEA, liver function tests, coagulation profile, |
|
complete blood count |
Chest X-ray: |
To rule out metastatic disease |
Multislice or multidetector |
To assess resectability; relationship of the tumor to the right |
abdominal CT: |
lateral wall of the SMA is the most critical aspect of the |
|
staging evaluation |
Endoscopic ultrasound |
Indicated if a low-attenuation pancreatic head mass is not |
scan (EUS): |
visualized on CT |
EUS-guided fine needle |
Needed to establish a tissue diagnosis which is necessary in |
aspiration (FNA): |
patients being considered for protocol-based, preoperative |
|
systemic therapy and/or chemoradiation; if no neoadjuvant |
|
therapy is indicated, a preoperative biopsy only delays |
|
exploration |
ERCP: |
To allow endobiliary decompression when operative inter- |
|
vention is delayed (poor performance status, need for |
|
further investigation of medical co-morbidities, or for |
|
neoadjuvant therapy). |
Mechanical bowel preparation
Prophylactic IV antibiotics prior to operation

Resection for Neoplasms of the Pancreas |
765 |
|
|
|
|
|
Procedure: Pancreatoduodenectomy |
|
|
|
|
STEP 1 |
Isolation of the infrapancreatic SMV |
|
|
|
|
We use a bilateral subcostal or midline incision, usually performing laparoscopy prior to laparotomy under the same anesthetic. Exposure is optimal with a Thompson retractor or another self-retaining retractor. When opening the abdomen, preserve the falciform ligament for later coverage of the stump of the gastroduodenal artery (GDA) prior to abdominal closure.
Evaluation for extrapancreatic metastatic disease:
■Biopsy-proven liver or peritoneal metastases are a contraindication to pancreatoduodenectomy
■Intraoperative ultrasonography of the liver is used selectively in patients whose CT is indeterminate
■Lymph node biopsy for frozen-section analysis remains controversial. In a good-risk patient with localized, resectable pancreatic cancer, lymph node metastases are not an absolute contraindication to pancreatoduodenectomy when performed as part of a multimodality approach to pancreatic cancer with an oncologic-type node dissection, not just “berry picking.” In a high-risk patient (medical comorbidities or oncologic concerns), a grossly positive regional lymph node is a contraindication to pancreatoduodenectomy.
First, enter the lesser sac by elevating the greater omentum off the transverse colon.
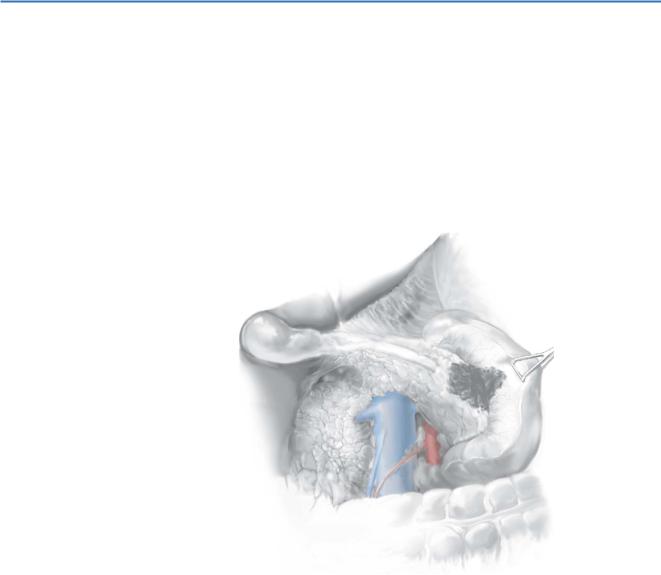
766 |
SECTION 6 |
Pancreas |
|
|
|
|
|
STEP 1 (continued) |
Isolation of the infrapancreatic SMV |
|
|
|
■ |
Mobilize the right colon and hepatic flexure to expose the entire duodenal sweep. |
|
|
|||
|
|
This step mobilizes the root of the small bowel mesentery by incising the visceral |
|
|
|
peritoneum to the ligament of Treitz. |
|
|
■ |
Incise the retroperitoneal peritoneum along the inferior border of the pancreas from |
|
|
|
the patient’s left of the middle colic vessels toward the patient’s right to expose the |
|
|
|
junction of the middle colic vein and the SMV. |
|
|
■ |
Divide the middle colic vein prior to its junction with the SMV to allow greater expo- |
|
|
|
sure of the infrapancreatic SMV; this minimizes the risk of traction injury to the |
|
|
|
SMV. |
|
|
■ |
We do not attempt to develop a plane of dissection between the anterior surface of |
|
|
|
the SMV and the posterior surface of the pancreatic neck as the pancreatic head |
|
|
|
neoplasms involve the posterolateral aspect of the SMV or SMPV confluence not the |
|
|
|
anterior surface of these vessels. We rarely divide the gastroepiploic vein at this time |
|
(unless it is coming off of a common trunk with the middle colic vein); this vessel is much easier to divide after the pancreatic transection.
STEP 2 |
Extended Kocher maneuver |
|
Begin the Kocher maneuver at the transverse portion of the duodenum by dissecting the |
|
|
|
inferior vena cava (IVC). |
|
Elevate all fibrofatty and lymphatic tissue medial to the right ureter and anterior to |
|
the IVC with the specimen (we usually preserve the gonadal vein as it courses anterior |
|
to the right ureter because it is a good landmark to avoid inadvertent injury to the right |
|
ureter). |
|
Fully mobilize the pancreatic head and duodenum to the cephalad aspect of the left |
|
renal vein as it crosses the aorta. The SMA origin can often be exposed at this level by |
|
medially rotating the pancreatic head and duodenum and incising the perineural tissue |
|
lateral to the first 2cm of the SMA origin. |
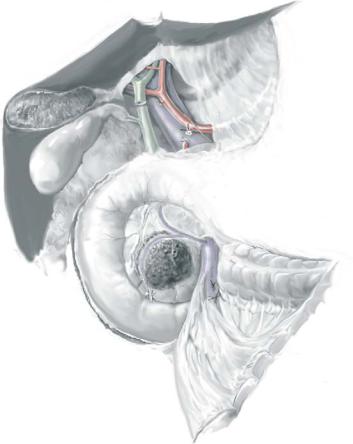
Resection for Neoplasms of the Pancreas |
767 |
|
|
|
|
STEP 3 |
Dissection of the porta hepatis |
|
|
|
|
Expose the common hepatic artery (CHA) by removing the lymph node that lies directly anterior to the CHA proximal to the right gastric artery and the GDA; the portal vein (PV) lies posterior to the inferior border of the CHA just proximal to the GDA origin.
Ligate and divide the right gastric artery and the GDA. If the tumor extends to within a few millimeters of the GDA origin, do not attempt bluntly to dissect the origin of the GDA; instead, obtain proximal control of the CHA and distal control of the proper hepatic artery and then divide the GDA flush at its origin. The hepatic artery is fragile; aggressive blunt dissection may create an intimal flap. We close the arteriotomy at the GDA origin with 6-0 polypropylene suture often with a small vascular pledget.
Perform cholecystectomy and transect the common hepatic duct (CHD) at its junction with the cystic duct, thereby exposing the underlying PV. Careful palpation prior to division of the bile duct should alert one to the possibility of a replaced right hepatic artery coming off the SMA traveling posterior to the lateral aspect of the bile duct. Note: if the foramen of Winslow was closed due to adhesions, it should have been reestablished at the time of the Kocher maneuver. Access to the foramen of Winslow is necessary to palpate the porta hepatis and appreciate a replaced right hepatic artery.
When possible, place a gentle bulldog clamp on the transected CHD to prevent bile spillage until the bile duct reconstruction.
Divide the loose connective tissue anterior to the PV caudally to the junction of the PV and the neck of the pancreas. The PV should not be extensively mobilized at this point, as iatrogenic injury to the PV prior to gastric and pancreatic transection results in excessive blood loss due to inadequate vascular exposure.
Be aware of anomalous hepatic arterial circulation. Rarely, the hepatic artery (distal to the origin of the GDA) courses posterior to the PV. An accessory or replaced right hepatic artery may also course posterolateral to the PV but would arise from the proximal SMA, not the celiac axis. The right hepatic artery should be preserved. The entire common hepatic artery may also arise from the SMA.
768 |
SECTION 6 |
Pancreas |
|
|
|
STEP 4 |
Transection of the gastric antrum (or the duodenum if pylorus preservation |
|
|
is planned) |
|
|
|
Ligate and divide the terminal branches of the left gastric artery along the lesser curvature of the stomach prior to gastric transection.
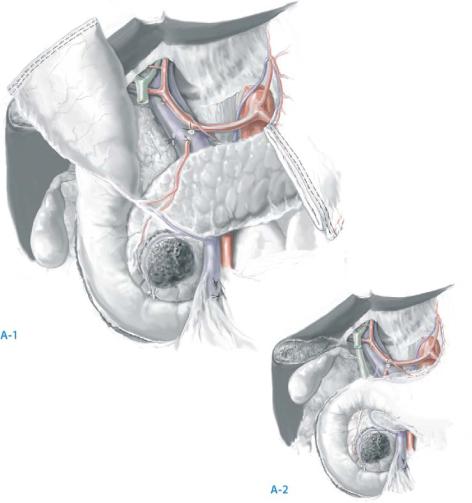
Transect the antrum of the stomach with a linear cutting stapler at the third or fourth transverse vein on the lesser curvature and at the confluence of the gastroepiploic veins on the greater curvature (A-1).
Divide the omentum at the site of transection of the greater curvature transection. Pylorus preservation may be considered in patients with small periampullary
neoplasms. It should not be performed in patients with bulky neoplasms of the pancreatic head, neoplasms involving the first or second portions of the duodenum, or lesions associated with grossly positive pyloric or peripyloric lymph nodes (A-2).
Divide the gastroepiploic arcade and the duodenum at least 3cm beyond the pylorus whenever possible. However, at the time of reconstruction, we trim another 1.0–1.5cm off of the duodenum to create the duodenojejunostomy 1.0–1.5cm from the pylorus to ensure an adequate blood supply to the duodenum.
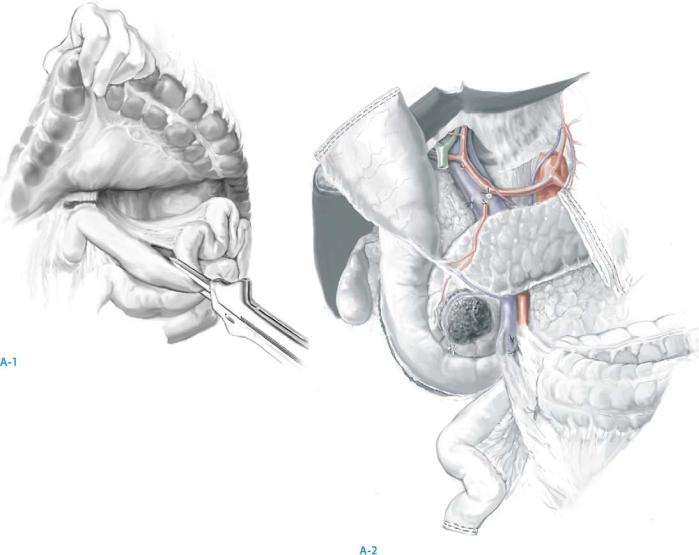
Resection for Neoplasms of the Pancreas |
769 |
|
|
|
|
STEP 5 |
Transection of the jejunum |
|
|
|
|
Take down the loose attachments of the ligament of Treitz with care to avoid the inferior mesenteric vein
Transect the jejunum with a linear cutting stapler about 10cm distal to the ligament of Treitz, and ligate and divide the mesentery (A-1). We prefer to tie the vessels on the mesenteric side (staying side) and use the harmonic scalpel on the serosal (jejunal/duodenal) side.
Continue this dissection to involve the fourth and third portions of the duodenum. Reflect the devascularized duodenum and jejunum beneath the mesenteric vessels (A-2).
770 |
SECTION 6 |
Pancreas |
|
|
|
STEP 6 |
Transection of the pancreas and completion of the retroperitoneal dissection |
|
|
|
|
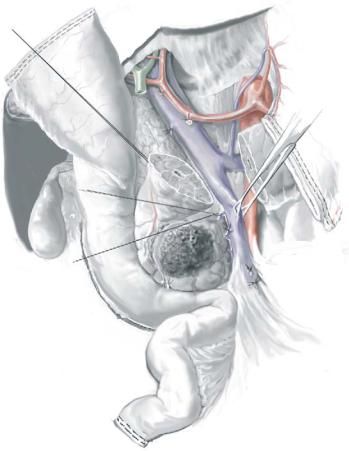
The most important and difficult part of the operation involves complete mobilization of the SMPV confluence and separation of the specimen from the right lateral border of the SMA.
Place traction sutures on the superior and inferior borders of the neck of the pancreas. This is important along the inferior border of the pancreas, where a small artery will be found in most patients.
Transect the pancreas with electrocautery down to the anterior surface of the SMPV confluence.
If there is evidence of tumor adherence to the PV or SMV, the pancreas can be divided at a more distal location (along the left or medial border of the SMPV confluence) in preparation for segmental venous resection.
Reflect the specimen to the patient’s right and separate it from the PV and SMV by ligation and division of the small venous tributaries to the uncinate process and pancreatic head. Note: The relationship of the tumor to the lateral and posterior walls of the SMPV confluence can be directly inspected only after gastric and pancreatic transection. This relationship cannot be accurately assessed intraoperatively by simply developing a plane of dissection between the anterior surface of the SMPV confluence and the posterior aspect of the neck of the pancreas (which is why this age-old maneuver is no longer performed earlier in the operation).
