
clavien_atlas_of_upper_gastrointestinal_and_hepato-pancreato-biliary_surgery2007-10-01_3540200045_springer
.pdf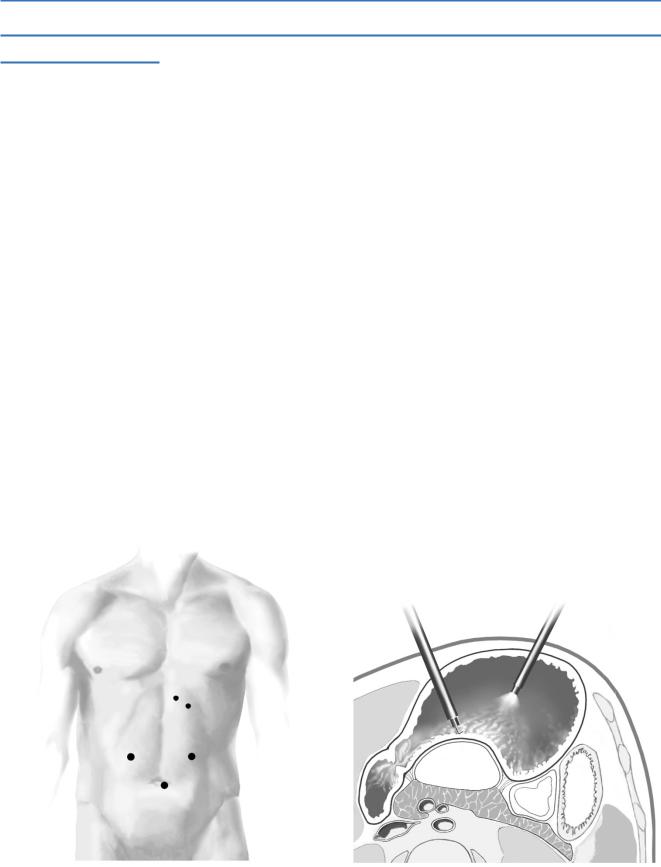

Drainage of Pancreatic Pseudocysts |
741 |
|
|
STEP 1 (continued)
Next, shut off gas insufflation into the peritoneal cavity and open the valves on these trocars to room air to allow any small amount of gas that might leak from inside the stomach around the gastric trocars to escape instead of accumulating in the peritoneal cavity and competing for space with the distended stomach. The insufflation pressure in the gastric lumen should be set at 20cm/H2O with insufflation connected to one of the trocars.
It is usually possible to see a convexity on the posterior gastric wall created by the pseudocyst. To localize the pseudocyst, pass a long aspirating needle through the back wall of the stomach into the cyst and aspirate some cyst fluid. If fluid is not found, either the needle is not in the cyst or the cyst contents are too thick to aspirate. The latter is uncommon, however, so the first alternative is most likely. Check the CT, recalculate the cyst location, and try again. In our experience, finding the cyst has rarely been difficult and has never required use of laparoscopic ultrasonography; however, use of this technique may prove helpful.
Having located the cyst, use the hook monopolar electrocautery (at a high power setting) to cut a hole through the posterior gastric wall into the pseudocyst; do not make the full cystogastrostomy incision, just a 1-cm hole (A-3).
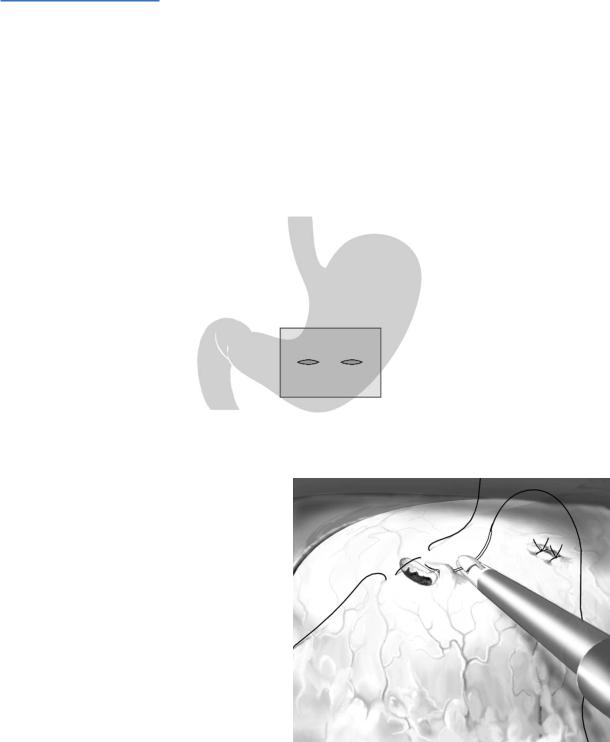
Next, aspirate the cyst contents and pass the scope into, or almost into, the cyst cavity. After determining where your entry hole lies in relation to the cyst’s circumference and area of contact with the stomach, extend the cystogastrostomy incision so it involves the center of the common wall between cyst and stomach and spans one-third to one-half of the cyst diameter. Check for bleeding and control with further electrocautery or suture ligation.
Remove semi-solid necrotic debris from within the cyst lumen and temporarily deposit this material in the dependent fundus of the stomach; the debris should be pushed through the pylorus into the duodenum before removing the gastric trocars. Do not try to “debride” the wall of the pseudocyst.
A-3
Drainage of Pancreatic Pseudocysts |
743 |
|
|
Postoperative Care
■Observation in the Intensive or Intermediate Care Unit is required only in selected patients.
■Nasogastric suction for 48h.
Local Postoperative Complications
■Early:
–Upper GI bleeding – usually from the anastomotic site
–Anastomotic leak and/or intra-abdominal abscess
–Beware alcohol withdrawal
■Late:
–Recurrent pseudocyst
–Error in diagnosis, i.e., mistaking a cystic neoplasm of pancreas for a pseudocyst; this leads to a persistent recurrent cyst with symptoms

744 |
SECTION 6 |
Pancreas |
|
|
|
|
Tricks of the Senior Surgeon |
|
Open internal drainage of pseudocysts:
■Always make sure that the cyst contents are that of a pseudocyst; i.e., clear, opalescent or brownish and not mucoid as in a cystic neoplasm.
■Leave the aspirating needle in place to enter the cyst with a knife alongside the needle.
■Use a right-angled clamp to elevate the cyst wall to make an adequate sized opening.
■Virtually always biopsy the cyst wall.
■If the cyst contents appear purulent, send the fluid for gram stain. If only white cells are seen, then internal drainage can be done; however, if numerous bacteria are seen and the fluid is purulent, external drainage is preferable.
■Use intraoperative ultrasonography liberally, because it can identify and locate more than one cyst. It can also locate the common bile duct and pancreatic duct. Finally, it can reveal the relationship of the pseudocyst to adjacent visceral and vascular structures.
Laparoscopic cystogastrostomy:
■This laparoendoscopic approach recapitulates the open transgastric cystogastrostomy. While others have described a side-to-side cystogastrostomy via a laparoscopic approach using a linear endoscopic stapler, the risk of an intraperitoneal “anastomotic leak” is avoided by the approach we have described.
■The initial short cystogastrostomy can be lengthened using an endoscopic linear stapler or even the harmonic scalpel, but we have not found this necessary and these techniques just increase the cost of the procedure.
■Carefully inspect the site of the cystogastrostomy; if there is any bleeding, the incision can either be reefed/oversewn intragastrically via the two trocars using a 2-0 silk suture or further controlled with electrocautery.
■Beware of the patient with a large “pseudocyst” of the pancreatic body after an episode of necrotizing pancreatitis. Be certain to exclude the presence of splenic vein compression/occlusion and gastric varices; these varices can be quite large and lead to severe hemorrhage during the cystogastrostomy.

|
Denervation: Pain Management |
|
Michael G. Sarr, Keith D. Lillemoe (Intraoperative Chemical Splanchnicectomy), |
|
Bhugwan Singh, J.E.J. Krige, Philip C. Bornman (Thoracoscopic Splanchnicectomy) |
|
Introduction |
|
The pain of both benign and malignant pancreatic disease can be incapacitating. |
|
Attempts to relieve pancreatic pain by extensive operative denervation have proved not |
|
to be effective. Newer, less-invasive techniques of selective denervations appear effective |
|
as shorter medium-term palliation. |
|
Indications and Contraindications |
|
■ Epigastric and back pain related to: |
Indications |
|
|
unresectable pancreatic, periampullary, and other upper GI cancers, |
|
chronic pancreatitis not amenable to other operative therapy |
|
■ Non-response to other attempts at percutaneous or endoscopic chemical |
Contraindications |
|
|
splanchnicectomy |
746 |
SECTION 6 |
Pancreas |
|
|
|
|
Procedures |
|
Intraoperative Chemical Splanchnicectomy
Michael G. Sarr, Keith D. Lillemoe
Intraoperative chemical splanchnicectomy can be useful, especially in patients who are found at the time of exploration for resection to have unresectable pancreatic cancer. Rather than having these patients undergo percutaneous or endoscopic chemical splanchnicectomy postoperatively, an intraoperative approach is easy, effective, and warranted.
Procedure
■The celiac plexus contains visceral afferent (pain) nerves from the stomach, pancreas, hepatobiliary tree, kidneys, and mid gut.
■There are one to five ganglia on each side of the celiac and superior mesenteric arteries which lie anterior to the diaphragmatic crura and medial to the adrenal glands.
■Supplies needed include a 10or 20-ml syringe, a 20-gauge spinal needle, and 40ml of a 50% alcohol or 5% phenol solution.
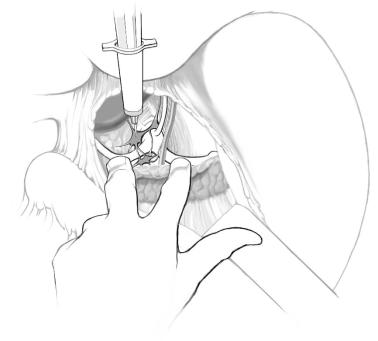
■The lesser curvature of the stomach is retracted caudally.
■The first two fingers of the surgeon’s left hand “straddle” the aorta.
■The index finger palpates the pulse of the splenic artery, while the second finger palpates the thrill of the common hepatic artery.
■The surgeon’s right hand controls the syringe with the neurolytic agent.
■The spinal needle is advanced into the right para-aortic region just rostral to the hepatic artery, and is clamped by the assistant to prevent displacement.
■The surgeon aspirates the syringe; if no blood is obtained (i.e., needle in vessel), 10ml of the neurolytic agent is injected, the needle removed, and the area compressed with a gauze pack.
■The syringe is re-filled, and the same maneuver is carried out just below the common hepatic on the right para-aortic area and rostral and caudal to the splenic artery in the left para-aortic region.
Denervation: Pain Management |
747 |
|
|
Thoracoscopic Splanchnicectomy
Bhugwan Singh, J.E.J. Krige, Philip C. Bornman
STEP 1
With the introduction of minimal access thoracostomy, interest and experience with a thoracoscopic approach to splanchnic nerve transection has grown. This procedure avoids the need for a major thoracotomy.
A standard general anesthetic is administered using a single lumen endotracheal tube.
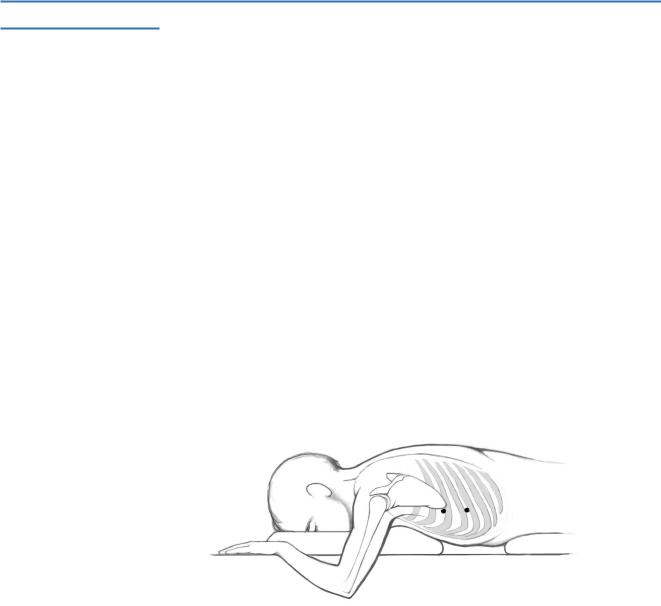
The patient is positioned in a prone position; pillows support the epigastric and sternal areas to facilitate breathing.
The arms are abducted and elbows flexed.
A pneumothorax is induced using a Veress needle placed in the intercostal space adjacent to the inferior scapular angle (usually 5th intercostal space); the pneumothorax is maintained with carbon dioxide insufflation to an intrapleural pressure of 8cm water; total lung collapse is not necessary because an 8 cm pressure pneumothorax is adequate to visualize the splanchnic nerves.
A 5-mm cannula is introduced in the 7th intercostal space in the posterior axillary line; a laparoscope/”thoracoscope” is passed through this cannula, connected to a video monitor, and is passed into the pleural space.
A second 5-mm cannula, inserted at the site of Veress needle placement, provides access for the dissecting instrument.
On the right side, the procedure commences at the 4th intercostal space at the level of the azygous vein, which is usually the origin of the most proximal splanchnic root.
748 |
SECTION 6 |
Pancreas |
|
|
|
STEP 2
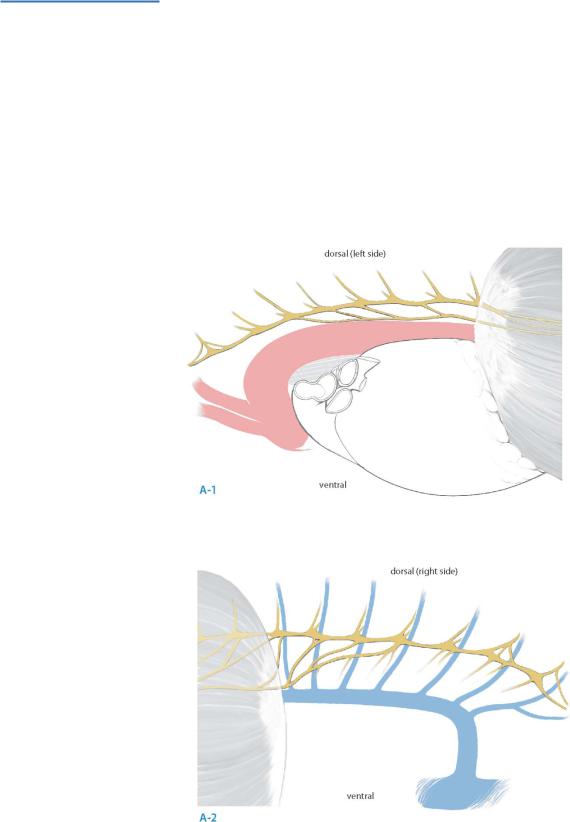
Accurate identification of the splanchnic nerves is the critical initial step in this procedure (A-1, A-2).
Splanchnic nerves [greater (GSN), lesser (LSN) and least (lsn)] have their origin medially from the sympathetic chain, usually from the lower eight ganglia.
Whereas the GSN is consistently present, presence of the LSN and the lsn is more variable, ranging from 86% to 100% and from 16% to 98% respectively.
The GSN is usually formed by contributions from the 5th to 9th ganglia, but contributions range widely from the 3rd to 11th thoracic ganglia.
Variable intersplanchnic connections occur between the splanchnic roots and nerves and provide an alternate neural pathway for transmission of pancreatic pain.

Denervation: Pain Management |
749 |
|
|
STEP 3
Using an electrosurgical hook, the parietal pleura is entered approximately 10mm medial to the sympathetic chain; the pleura is stripped off the posterior chest wall up to the diaphragmatic recess so that a 10-mm-wide longitudinal pleurotomy is achieved.
Commencing from the most proximal contribution to the GSN and working distally, all branches passing medially from the sympathetic chain are mobilized sequentially using the hook dissector and transected using cautery; in this fashion all roots to the GSN, LSN and lsn (if present) are transected. When there is any doubt, gentle traction on the sympathetic chain confirms complete dissection of splanchnic nerves.
On the left side, the procedure commences at the 4th intercostal space at the level
of the aortic arch, usually the origin of the most proximal splanchnic root; the principles of this technique are the same as on the right side.
Extension of peri-pancreatic inflammation to the retropleural space may cause thickening of the pleura, impairing visualization and impeding easy dissection of the sympathetic chain and splanchnic nerves.
Troublesome bleeding can be controlled by judicious use of cautery.
After completion of the neurotomy, carbon dioxide insufflation is stopped and gentle suction applied through the dissecting port with positive end expiratory pressure applied by the anesthetist.
Intercostal chest drains are not placed, provided lung reexpansion is present up to the intercostal musculature.
A chest radiograph is taken when respiratory distress, progressive desaturation, or unremitting surgical emphysema develops.
The patient may be discharged after overnight hospital stay.
