
clavien_atlas_of_upper_gastrointestinal_and_hepato-pancreato-biliary_surgery2007-10-01_3540200045_springer
.pdf
644 |
SECTION 4 |
Biliary Tract and Gallbladder |
|
|
|
STEP 2 |
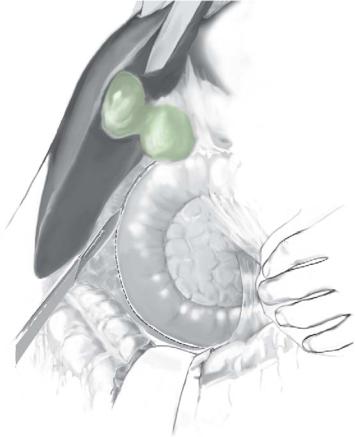
Kocherization and mobilization of porta-hepatis |
|
|
|
|
A full kocherization is performed to allow access to the lower bile duct and a retropancreatic approach.
Operative Treatment of Choledochal Cysts |
645 |
|
|
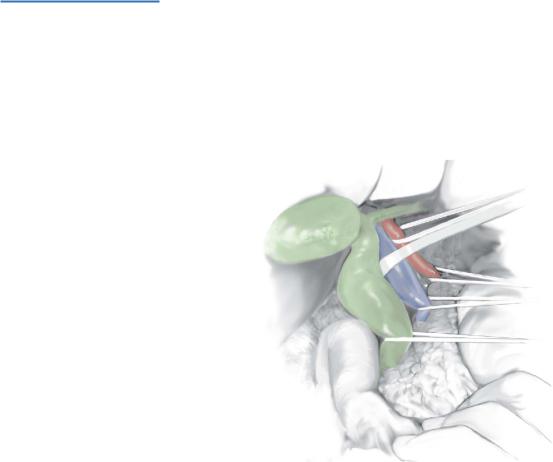
STEP 3
The cyst is now mobilized inferiorly to its lowest extent into the head of the pancreas avoiding damage to the pancreatic duct.
Intraoperative ultrasound may have provided useful information in this regard. Ultrasonic dissection (CUSA) may aid in dissecting the cyst from the pancreas. Multiple small vessels may be divided with diathermy.
646 |
SECTION 4 |
Biliary Tract and Gallbladder |
|
|
|
STEP 4 |
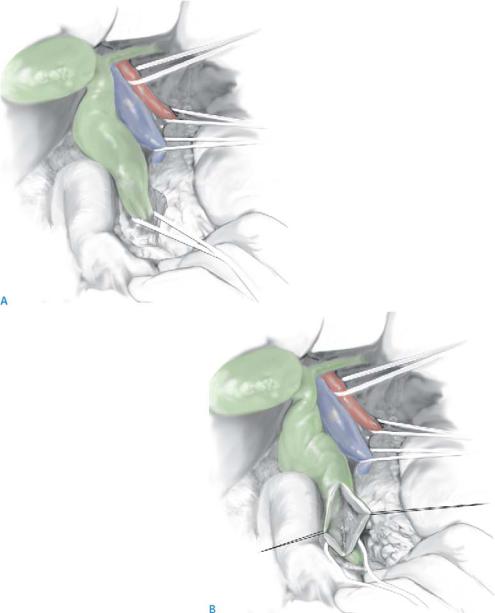
Opening the choledochal cyst |
|
|
|
|
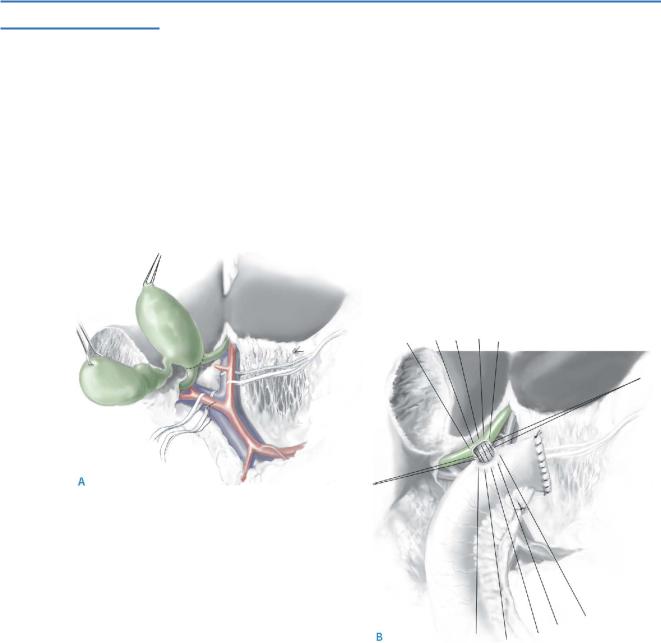
The cyst may require to be opened to enable visualization of the pancreatic duct, at its insertion into the common bile duct.
The cyst is controlled at its lower end above the junction of the bile duct and the pancreatic duct, which is preserved carefully (A). A view is shown looking into the pancreas with a partly mobilized cyst (B).
Operative Treatment of Choledochal Cysts |
647 |
|
|
|
|
STEP 5 |
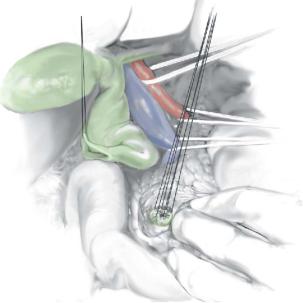
Repair of the distal common bile duct |
|
|
|
|
If residual cyst lining remains it can be cauterized cautiously with diathermy current. The lower common bile duct is repaired with interrupted 5-0 PDS sutures just at its
junction with the pancreatic duct, damage to which is avoided carefully. The divided distal duct is seen with the pancreatic duct on view and closure started.
648 SECTION 4 Biliary Tract and Gallbladder
STEP 6
The cyst is mobilized superiorly to the confluence of the right and left hepatic ducts. Assess carefully for aberrant ducts, which may need to be divided separately.
The dissection may need to be extended along the extrahepatic course of the left duct if it is ectatic.
Some advocate extended liver resection for cysts extending into the left or right duct. It is possible to anastomose to the cyst at the confluence of the ducts, if this extends
more proximally, rather than performing liver resection.
A hepaticojejunostomy is performed in an end-to-side manner with a single layer of interrupted 4-0 PDS sutures. A Roux-en-Y limb of 70cm of proximal jejunum should be used.
A drain should be left adjacent to the hepaticojejunostomy. The cyst is removed and the hepaticojejunostomy started (A, B).
Complications
Biliary Leak
■A small amount of bile drainage can be expected in the first 48hours, and will usually settle.
■Continuing leakage should be managed conservatively with drainage.
■Keep an eye open for amylase-rich fluid if there is difficult pancreatic dissection and/or persistent nonbile leak.
Late Complications
■ Cholangitis (20–40%) usually in association with an anastomotic stricture.

SECTION 5
Portal Hypertension
J. Michael Henderson

Introduction
J. Michael Henderson
Surgical operations for prevention of recurrent variceal bleeding have significantly diminished over the past decade. This section illustrates the different surgical options for managing variceal bleeding with total, partial, and selective shunts, and devascularization procedures. The peak of non-transplant surgical intervention for variceal bleeding was in the 1970s to 1980s when other management options were not available. What has happened since that time is that the pathophysiology has been better defined for portal hypertension, and there have been technological advances in endoscopy and radiology.
The pathophysiology of portal hypertension follows the sequences of an initial obstruction to portal venous flow, an increase in portal pressure, development of collaterals, splanchnic hyperemia, and a hyperdynamic systemic circulation. Pharmacologic intervention with non-cardioselective beta-blockade to moderate these pathophysiology changes has been successful in preventing initial bleeding, and in reducing the risk of re-bleeding. Reduction of splanchnic hyperemia with somastostatin or its analogues is effective in the acute setting.
Endoscopy has dramatically improved in the last two and a half decades with the universal adoption of videoendoscopy. For managing esophageal varices, treatment has evolved from an almost blind stick of a bleeding esophageal varix of three decades ago to sophisticated multiband-like ligators for both acute and elective management of gastroesophageal variceal bleeding. The lower complication rates with banding
as opposed to sclerotherapy have established this as the primary treatment option for acute bleeding and initial prevention of recurrent bleeding.
The radiological evolution of hepatic imaging and ability to access the portal vein has led to transjugular intrahepatic portal systemic shunts (TIPS) over the past decade. TIPS can decompress varices successfully. High stenosis rates with bare stents have been reduced with the evolution of covered stents, but pseudointimal hyperplasia does remain a problem with a requirement for long-term monitoring of TIPS to maintain decompression.
Liver transplantation has also evolved dramatically over the last two decades, becoming the main surgical option for patients with variceal bleeding and advanced liver disease, and the only therapy that has significantly impacted mortality.
For these patients, liver transplant is the best treatment option.
Is there still a role for the operations for portal hypertension described in this section? These procedures may be useful in some patient populations and are among the treatment options in different parts of the world. It should be recognized that all of the operations described in this section can work very well for some patients. While there is no doubt that the overall use of these procedures has markedly diminished, they should not be lost from the repertoire of surgeons managing complex hepatobiliary disease and should remain part of the repertoire of liver transplant surgeons where there is refractory variceal bleeding with well-preserved liver function. This chapter presents excellent descriptions of these operations from recognized experts with technical details and tips that make the operations possible for other surgeons with expertise in hepatobiliary surgery.

654 |
SECTION 5 |
Portal Hypertension |
|
|
|
|
|
|
Investigation/Preparation |
|
|
|
■ |
Focus on the varices, the vasculature, and the underlying liver disease. |
|
|
■ |
Clinical picture: recurrent variceal bleeding that is refractory or not amenable to |
|
|
|
appropriate endoscopic therapies. |
|
|
■ |
Laboratory studies to calculate Child’s classification. |
|
|
■ |
Radiologic workup with vascular ultrasound, but arteriography is advisable in |
|
|
|
reaching a final decision to proceed with DSRS. Superior mesenteric and splenic |
|
|
|
arteriography followed through the venous phases defines portal venous anatomy |
|
|
|
and collaterals. The left renal vein should be directly studied because 20% of the |
|
|
|
population have abnormal left renal veins. |
|
|
■ |
Timing and preparation: |
|
|
|
– DSRS should not be performed as an emergency procedure, but patients stabilized |
|
and brought to elective operation.
– Ascites should be diuresed with appropriate salt restriction and diuretics prior to surgery.
– Nutritional status should be optimized.
– Coagulation status should be corrected.
– Preoperative and perioperative fluid management is important, particularly with limitation of free sodium. Patients should be run “dry” to minimize the risk of postoperative ascites.
– Appropriate perioperative antibiotic prophylaxis should be used.
