
clavien_atlas_of_upper_gastrointestinal_and_hepato-pancreato-biliary_surgery2007-10-01_3540200045_springer
.pdf
Surgical Staplers
Nicolas Attigah, Markus Schäfer
Introduction
Stapling devices belong to the standard repertoire of modern gastrointestinal surgery, especially since the successful advent of minimally invasive surgery. Therefore, a thorough knowledge and safe handling of stapling devices need to be acquired early during surgical training.
History
Based on the main principles of mechanical stapling, (Table1) Hültl from Budapest, Hungary, developed the first linear stapler in 1909 to close the remnant stomach during gastrectomies. The handling of that instrument was hampered by its weight and bulk. Petz, another Hungarian surgeon, and Friedrich and Neuffer from Germany created lighter and more convenient stapling instruments during the 1920s. Driven by the lack of surgeons after World War II, the Russian government encouraged the development of different mechanical devices for linear and circular stapling to help less well-trained surgeons to safely perform standardized surgical procedures, e.g., gastrectomies and bowel resections.
In the 1960s, the American surgeon Ravitch brought those instruments to the United States and focused on their improvement in terms of applicability and reliability. In partnership with industry, preloaded plastic cartridges, double-staggered staple lines, and different lengths of staple lines were developed. Since the mid-1970s, single-patient- use stapling devices have become widespread worldwide.
The success of minimally invasive surgery promoted the development of miniaturized stapling devices during the past decade; such devices are now used routinely in many different operations.

28 SECTION 1 General Principles
Types of Mechanical Staplers
There are currently three major types of mechanical stapling devices in clinical use for open and laparoscopic surgery. As described in Tables1 and 2, the principles and prerequisites of mechanical stapling remain largely unchanged.
Table1. Principles of mechanical stapling
Tissue compression
Tissue stapling using metallic wire as staples
Configuration of the closed staples in B-shape
Staggered positioning of the staple lines
Table2. Aims of surgical stapling
Creation of an adequate lumen
Preserving adequate tissue vascularization
Preventing tension of adapting tissues
Avoiding leakage and fistula formation
Provision of good hemostasis
Mechanical reliability/uniformity of stapling devices
Linear Stapler or TIA Stapler (Transverse Anastomosis Stapler)
Linear staplers are predominantly used to close the ends of a hollow organ or vessel. They are claimed to give easier access to narrow anatomic sites such as the pelvis. For such applications, linear staplers with articulated heads and flexible shafts have been developed. These staplers normally apply two lines of staples that are staggered to maximize local blood supply. Vascular linear staplers apply three staggered lines of staples to achieve tight closure of the vessel. The staple height is either fixed or, with some brands of stapler, can be “adjusted” during the application. For most applications, the use of a fixed staple height is preferred. Because linear staplers are mainly used to close open organs, they do not include a cutting device.
The length of the stapler lines varies between 30mm and 90mm, while the height of individual staples varies from 2.5mm to 4.8mm, depending on the tissue to be stapled (e.g., vascular staples = 2.5mm; staples for normal intestinal tissue = 3.5mm; for stomach or thicker tissues = 4.8mm).
3 lines of staplers
2 lines of staplers

Surgical Staplers |
29 |
|
|
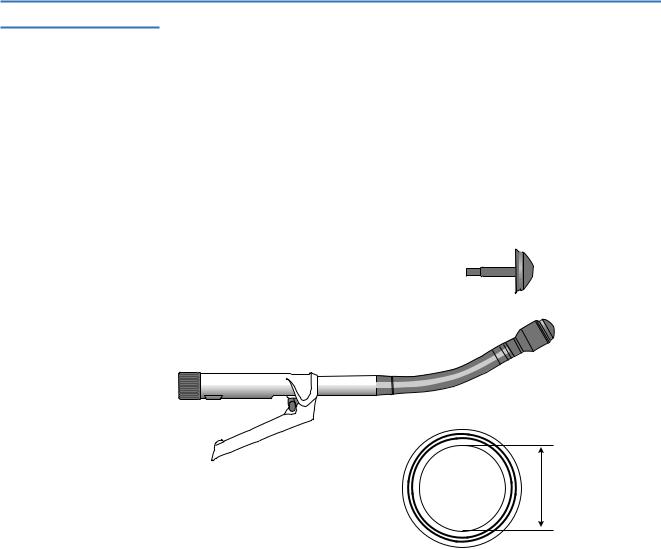
Linear Cutter or GIA Stapler (Gastrointestinal Anastomosis Stapler, A)
These staplers are basically linear staplers with an integrated cutting device. Four staggered lines of staples (two “rows”) are applied, and the tissue between the two inner staple lines is transected. The main indications are transecting and stapling closed both ends of a hollow organ (e.g., bowel, bronchus) or vascular structure. In addition, because of the two separate rows of staples, side-to-side anastomoses can be created. The staple height is fixed and must be chosen before using the instrument in accordance to the type of tissue. Different staple sizes and wire diameters are available in preloaded, single-use cartridges.
The length of the staple lines varies between 55mm and 100mm for open surgery. Specially designed linear cutters have been developed for minimally invasive
surgery (B). Articulated heads are often used to overcome angulations related to the trocar positioning.
10 |
9 |
8 |
7 |
6 |
5 |
4 |
3 |
2 |
1 |
0
|
|
4 stagered |
A |
|
lines of staplers |
B
30 SECTION 1 General Principles
Circular Stapler
These staplers apply two staggered circular lines (one “row”) of staples. The cutting device transects the tissue inside the staple lines. The staple height is also variable, depending on the tissue thickness. Circular staplers are used mainly for end-to-end and end-to-side anastomoses of the esophagus, stomach, and rectum.
Circular staplers are available with various diameters, ranging from 21mm to 33mm. It should be noted that the true (inner) diameter of the created anastomosis is somewhat smaller (range 12.4–24.4mm). In order to avoid mucosal tears, sizers should be used to estimate the diameter of the respective hollow organ. The anvil must be secured by a proper purse-string suture which is applied intraluminally to create the anastomosis.

Surgical Staplers |
31 |
|
|
Staples
Design Type of Staplers (Table3)
The most commonly used staples have a rectangular shape and are preloaded in cartridges. The staples are pushed through the tissue under the pressure created by closing the stapler. Once they reach the anvil, the staples are buckled or bent into the final B-shape. The B-form allows both the firm connection and sufficient vascularization of the adapted tissue. Note that the “height” of the staples after the instrument is “fired” is smaller.
In order to achieve a safe anastomosis or closure, the staple height should be adapted to the thickness of the tissue. The staple height is indicated by different colors of cartridges. The majority of staplers in current use are equipped with a fixed staple height.
The staple height is fixed for linear cutters, whereas linear staplers and circular staplers may have somewhat variable staple heights that can be adapted intraoperatively, according to the type of tissue.
Table3. Staple characteristics and applications
Cartridge type |
Vascular |
Standard |
Thick |
|
|
|
|
Color |
White |
Blue |
Green |
Staple height |
Fixed |
Fixed |
Fixed |
Before B formation |
2.5mm |
3.5mm |
4.8mm |
After B formation |
1.0mm |
1.5mm |
2.0mm |
Applications |
Thin tissues |
Esophagus |
Rectum |
|
Well-vascularized tissues |
Small bowel |
Stomach |
|
Vessels |
Large bowel |
Bronchus |
|
Pancreas |
Lung |
|
|
Liver |
|
|
|
|
|
|

32 SECTION 1 General Principles
Technical Aspects
Whereas at the beginning of the stapler era, staples were made of silver and steel, current stapler technology uses titanium. Titanium has better biocompatibility and causes fewer artifacts during CT and MRI. Furthermore, titanium is not affected by static magnetic fields and shows only a minimal temperature rise during MRI.
The wire thickness has also been reduced to 0.2–0.3mm.
Tricks of the Senior Surgeon
■The surgeon must be familiar with the different types of stapling devices. Incorrect handling, not stapler misfunction, remains a major cause of failure.
■Stapling instruments should not be used on ischemic, necrotic, or markedly edematous or inflamed tissue, because tissue closure and anastomosis formation cannot be achieved safely.
■Adjacent adipose tissue from the serosal surface must be removed before firing the stapler.
■To prevent leakage and postoperative peritonitis, every anastomosis must be checked.

Principles of Drainage
Henrik Petrowsky, Stefan Wildi
Introduction
Drains are designated to evacuate intraperitoneal fluid collections. They can be used for diagnostic, prophylactic, or therapeutic purposes. In upper gastrointestinal surgery, diagnostic drains are mainly placed to assess intraperitoneal fluid collections in order to establish a diagnosis. These drains are seldom left in place and are, therefore, of minor importance. In contrast, prophylactic drains placed at the end of an operation are used frequently with two intentions: first, to prevent fluid accumulations which could be harmful (i.e., pancreatic juice or bile) or to evacuate fluid collections that can become infected and lead to the formation of intra-abdominal abscesses; second, prophylactic drains may be used to detect early postoperative complications, such as intra-abdominal bleeding or anastomotic leakage. Sometimes, fluid collections become infected and develop into abscesses; the management of these collections requires therapeutic drainage either by the percutaneous route or by reoperative surgical lavage.

34 SECTION 1 General Principles
Types of Drain
Drains can be divided into passive and active drains.
Passive Drains
Passive drains, such as the Penrose (A-1) and Easy Flow (A-2) devices, serve to evacuate fluid passively by providing a route of access secondary to the natural pressure gradients, such as gravity flow, muscle contraction, and overflow. The opening in the abdominal wall for these drains should be made large enough, because passive drains are potentially collapsible. Easy Flow drains have intraluminal corrugations to prevent complete collapse (inlay). Passive drains cannot be sealed and are open systems with the potential risk of retrograde infections. The advantages and disadvantages of open and closed-suction drains are outlined in Table 1.
A-1 |
|
A-2 |
A-1
A-2

Principles of Drainage |
35 |
|
|
Aktive Drains
Jackson-Pratt (A-1) and Blake (A-2) drains are commonly used radiopaque, silicone products for closed-suction systems. The Jackson-Pratt drain is oval-shaped with numerous orifices and intraluminal corrugations (inlay). The Blake drain has four channels along the sides with a solid core center. In contrast to passive drains, active or suction drains maintain a negative pressure gradient.
A-1 |
|
A-2 |
A-1
A-2
Sump Drains
Sump drains are usually double-lumen tubes with a larger outflow lumen and a smaller inflow “sump” lumen. The larger lumen is connected to a suction system and evacuates intra-abdominal secretions. The smaller lumen serves as a venting tube, allowing air to enter the larger lumen. This principle should help to break the vacuum in the large draining tube, maintaining the drain in a productive state, without the surrounding tissues continually occluding the drainage holes in the tube. Sump drains are often used when large fluid volumes have to be evacuated. The occlusion of the smaller venting tube by tissue debris due to retrograde inflow demonstrates a potential disadvantage of sump drains that occurs especially when the suction is disconnected. Some sump drains have an additional third lumen that allows the instillation of an irrigating solution.
Suction
1
Drainage
1

36 SECTION 1 General Principles
Complete Drainage System
Collapsible devices connected to the drain tubes automatically generate a negative pressure gradient and keep the system “sealed,” which is believed to result in a significant reduction of retrograde infections.
Table 1. Advantages and disadvantages of open and closed-suction drains
|
Open drain |
Closed-suction drain |
|
|
|
Advantages |
Generates pathways for bulky |
Lowers risk of retrograde infection |
|
or viscous material |
|
|
Lowers risk of mechanical |
Accurate measurement of drainage |
|
erosion and pressure necrosis |
|
|
|
Facilitates radiographic studies |
|
|
Skin protection from irritating discharge |
Disadvantages |
Retrograde infection |
More vulnerable to obstruction |
|
|
by small tissue fragments or ingrowth |
|
|
of surrounding tissue |
|
|
|
