
Practical Plastic Surgery
.pdf
302 |
Practical Plastic Surgery |
48
Table 48.1. Commonly used local flaps for chest wall defect repair
DefectSite |
Anterior,posteriorlateral, |
superiorintrathoracic |
Anterior,lateral |
Superior,posterior |
|
Anterior,intrathoracic |
Anterior |
Insertion |
Humerus |
|
Humerus |
Acromion,scapula, |
clavicle |
Medialscapula |
Costalcartilage5-7 |
Origin |
Vertebralspinespost |
iliaccrest |
Sternum,ribs,clavicle |
Occiput,ligamentum |
nuchae,vertebrae |
Ribs7-10 |
Pubissymphisis |
Pedicle |
ThoracordorsalA.post.perforators |
|
ThoracoacromialA.IMAperforators |
TransversecervicalA. |
|
LateralthoracicA.thoracodorsal branches |
SuperiorepigastricA |
MuscleFlap |
Latissimusdorsi |
|
Pectoralismajor |
Trapezius |
|
Serratusanterior |
Rectusabdominis |
Chest Wall Defects |
303 |
48
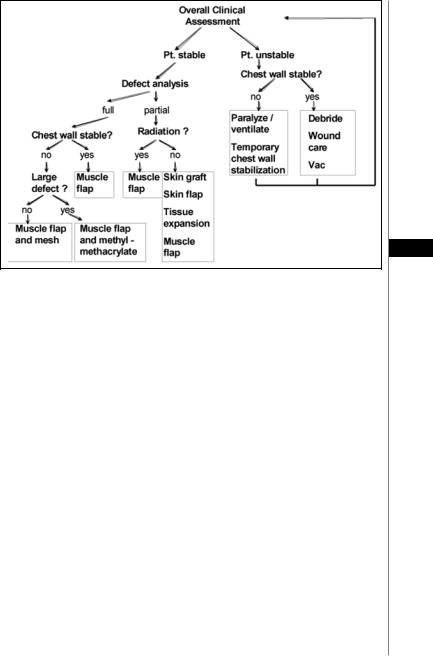
Figure 48.1. A management algorithm for the treatment of chest wall defects.
Pearls and Pitfalls
It is important to differentiate between traumatic chest wall wounds, usually the result of shotgun injuries, and surgical wounds due to resection of tumors or osteoradionecrosis. The trauma patient must be stabilized and serial debridements performed to establish the limits of viable tissue, while the surgical wound should be closed at the time of resection. It is useful to separate the reconstruction of the skeletal defect from the soft tissue problem; the former can almost always be addressed with polyethylene mesh and methyl methacrylate as a sandwich that is then sutured to adjacent remaining ribs. In smaller defects mesh alone can be used to stabilize the chest wall and support the overlying flap. Flap closure must be robust and airtight. The latissimus dorsi is really the workhorse of this region, either with a skin island or with an overlying skin graft. Intrathoracic defects, usually empyema cavities, pose a special challenge, requiring rib resection and control of bronchopleural fistulae for successful wound closure. Multiple muscle flaps may be needed in these cases.
Suggested Reading
1.Arnold PG, Pairolero PC. Chest-wall reconstruction: An account of 500 consecutive patients. Plast Reconstr Surg 1996; 98(5):804.
2.Chang RR, Mehrara BJ, Hu QY et al. Reconstruction of complex oncologic chest wall defects: A 10-year experience. Ann Plast Surg 2004; 52(5):471.
3.Cordeiro PG, Santamaria E, Hidalgo D. The role of microsurgery in reconstruction of oncologic chest wall defects. Plast Reconstr Surg 2001; 108(7):1924.
4.Losken A, Thourani VH, Carlson GW et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg 2004; 57(4):295.
5.Mansour KA, Thourani VH, Losken A et al. Chest wall resections and reconstruction: A 25-year experience. Ann Thorac Surg 2002; 73(6):1720.
6.Mathes SJ. Chest wall reconstruction. Clin Plast Surg 1995; 22(1):187.

Chapter 49
Coverage of Spinal Wounds
Jason Pomerantz and William Hoffman
Overview
As with other parts of the body, adequate management of wounds in the posterior midline of the trunk is based on fundamental principles of reconstructive surgery. These include general patient assessment (multisystem evaluation) and thorough, accurate defect analysis that guide the subsequent plan for timely, durable and safe wound closure.
Etiology
Wounds in the posterior midline have a few common etiologies. Most spinal wounds in adults occur after spine surgery and frequently involve the presence of foreign material (hardware). Midline back wounds, especially postsurgical wounds, are often infected, adding increased complexity to their management. Aside from infection, other factors such as a history of irradiation, chronic illness, malnutrition, and use of systemic steroids all complicate the management of spinal wounds. Pressure is also a contributing factor either solely or in combination with surgical manipulation. Any postsurgical spinal wound complication, such as dehiscence or purulent drainage, should be explored and debrided as soon as it is noted. Other causes of spinal wounds include traumatic soft tissue injuries and congenital anomalies such as spina bifida comprise.
Spina bifida is the most common birth defect of the central nervous system and involves incomplete fusion of the vertebrae dorsally. Types of spina bifida include meningocele (meninges only in the defect), myelomeningocele (meninges and spinal cord), syringomyelocele (meninges and spinal cord with increased fluid and pressure in central canal) and myelocele (absence of epithelial covering of spinal cord). Myelomeningocele is the most common, and initial treatment involves coverage of the spinal cord and meninges with well vascularized soft tissue in order to preserve spinal cord function and prevent future neurological sequelae. In contrast to post-surgical spinal wounds, closure of myelemeningocele defects is often straightforward and accomplished with skin flaps. In more difficult cases, other reconstructive options including muscle flaps are required in a cooperative effort between neurosurgeons, pediatric surgeons and plastic surgeons. In addition, prenatal surgical treatment of spina bifida is under investigation and may ultimately provide the best long term outcome by virtue of early correction of the defect.
Defect Analysis
After the etiology is determined, a description of the defect is made. The location (superior, middle or inferior) is the most important for determining which muscle flaps are most suitable for coverage. The spine can be divided into thirds
Practical Plastic Surgery, edited by Zol B. Kryger and Mark Sisco. ©2007 Landes Bioscience.
Coverage of Spinal Wounds |
305 |
based on the original scheme described by Casas and Lewis. The superior region extends from C3 to T7, middle from T7 to L1 and inferior from L1 to S5. The size of the wound should be noted as well as the presence of infection, necrotic tissue, exposed hardware, bone or dura.
Management
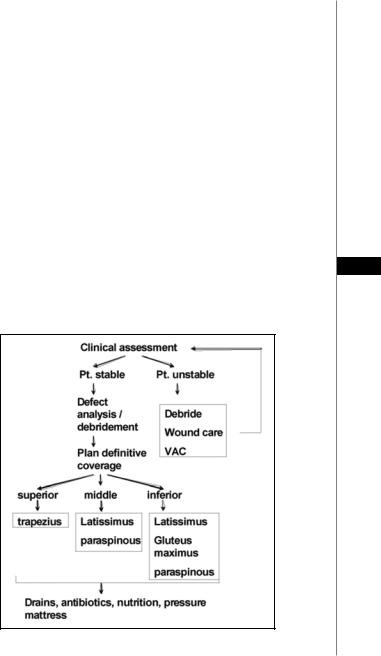
A general treatment algorithm for treating spinal wounds is shown in Figure 49.1. As with defects in other regions of the body, definitive closure of spinal wounds should take place as soon as the patient is medically stable. The patient’s nutritional status should be optimized prior to reconstruction. Adequate control of infection may require multiple debridements prior to wound closure. Treatment with systemic and local (beads) antibiotics is often implemented. Debridement in conjunction with the orthopedic or neurosurgery teams may include removal of spinous processes and various amounts of vertebral tissue in order to permit eradication of infection. Wounds should be cultured intraoperatively and antibiotic therapy tailored to treat specific organisms. During debridement, effort should be made to leave functioning hardware in place, as the ultimate goal is to achieve stable wound and hardware coverage, often attainable with the transposition of well-vascularized muscle flaps. The wound VAC offers a suitable option as a bridge to wound closure, either in systemically unstable patients or in situations that require multiple
debridements. The VAC is discussed in detail in the “Wound VAC” chapter. 49 Closure of postsurgical spinal wounds usually requires muscle as well as skin.
Table 49.1 lists some of the commonly used muscle flaps in spinal wound coverage. Healing by secondary intention, skin grafting or skin advancement are generally
Figure 49.1. A treatment algorithm for spinal wounds.
306
49
 Table 49.1. Commonly used local flaps for coverage of spinal wounds
Table 49.1. Commonly used local flaps for coverage of spinal wounds
Practical Plastic Surgery
WoundSite |
upper,middlelower |
middle,lower |
superior |
|
lower |
lower |
Insertion |
humerus |
posterior-medialribs |
acromion,scapula, |
clavicle |
greatertrochanter |
N/A |
Origin |
vertebralspinespost.iliaccrest |
spinousprocess,Iliaccrest |
occiput,ligamentumnuchae, |
vertebrae |
sacrum,ileum |
N/A |
Pedicle |
thoracordorsalA.post.perforators |
lumbarperforators |
transversecervicalA. |
|
superiororinferiorglutealA. |
leftorrightgastroepiploicA. |
Muscleflap |
LatissimusDorsi |
Paraspinous |
Trapezius |
|
Gluteusmaximus |
Omentum |

Coverage of Spinal Wounds |
307 |
considered inadequate for providing a durable repair, except in the most superficial wounds that do not contain hardware. Local muscle flaps that have adequate bulk and are well vascularized are available for all regions of the spine. Free tissue transfers are rarely required. In addition, there are few convenient recipient vessels in this region, and free tissue transfers often require pedicle extension with vein grafts.
Middle and Inferior Spinal Wounds
Several muscle flaps are used most frequently for coverage of spinal wounds. Flap choice is usually based on location of the wound. The paraspinous muscle flap can be used in the middle and inferior regions. This flap avoids a significant donor site defect and is based on posterior perforating vessels. The paraspinous flap is mobilized by releasing the fascia laterally, on either side of the wound. The muscle and fascia are then turned over or advanced into the midline to cover the wound and exposed hardware. Healthy skin flaps are advanced and closed on top of the muscle. Potential disadvantages of the paraspinous flap are that the muscle is often small and may have been partially debrided because of proximity to the wound. Furthermore, there is sometimes a need to debride overlying skin, making the skin closure difficult. In these cases, a skin graft may be required. Inferior spinal wounds, including those overlying the sacrum, can be covered with the gluteus muscle flap based on the superior and/or inferior gluteal vessels. Alternatively, the superior gluteal artery
perforator (SGAP) flap can be used in order to minimize donor site morbidity in 49 ambulatory patients.
Superior Spinal Wounds
The paraspinous muscles are particularly small and difficult to mobilize in the superior part of the back. In the superior region, the trapezius muscle flap is a good option. A skin paddle is designed over the distal portion of the trapezius, and the muscle is elevated off the deeper tissues and then divided lateral to its pedicle. The flap is then rotated into the wound. The donor site can usually be primarily closed. Division of the superior portion of the trapezius should be avoided to prevent postoperative shoulder drop. The latissimus dorsi flap is a commonly used muscle flap for spinal wound closure, appropriate for all regions of the spine. The latissimus can be used with a skin paddle or covered with a skin graft. This muscle can be rotated or advanced, based on the thoracodorsal pedicle. Alternatively, the latissimus flap may be based on posterior perforators after release from its attachment to the humerus and division of the thoracodorsal pedicle. This large muscle can be successfully transposed to cover most spinal wounds.
Finally, the omental pedicle flap has been used for coverage of spinal wounds, but is not a first choice given the availability of the muscle flaps described above, as well as the necessary creation of a lumbar hernia in order to pass the omentum from the abdominal cavity to the back.
Postoperative Considerations
Suction drains are usually placed at the time of surgery to prevent seroma formation at both the donor and recipient sites. These are left in place for variable periods of time depending on drain output as well as the surgeon’s preference. As a general rule, drains should not be removed until their output is almost zero.
Excess pressure on the flap is an important issue postoperatively, as many spinal wound patients are bedridden. Low pressure mattresses should be used to avoid flap ischemia. On the other hand, some surgeons prefer some mild pressure in line with

308 |
Practical Plastic Surgery |
the thinking that it decreases seroma formation. Usually, patients are kept prone or in the lateral decubitus position for an extended period of time (up to 3 weeks).
For infected spinal wounds, especially those with hardware in place, a prolonged course of IV antibiotics is generally used. Although not well studied for spinal wounds in particular, 6 weeks of organism-specific IV antibiotics after debridement and muscle flap coverage is a common practice, in accord with the antibiotic treatment of osteomyelitis in other regions of the body.
Pearls and Pitfalls
In cases involving bone, often with exposed hardware, muscle coverage is required to eliminate dead space and to combat infection. The most difficult area of the spine for obtaining muscle coverage is the lumbosacral region; the gluteus muscle is below and the latissimus above but neither reaches this area easily, and the paraspinous muscles end at this level as well. The gluteus remains the best choice for this area.
Hardware is always a difficult issue. In today’s complex spine cases, the hardware may be necessary to stabilize the spine after scoliosis repair or multiple level fusions. Any loose hardware must be removed and/or replaced. If there is severe infection (gross purulence) and loose hardware present, a body cast may be required while the wound heals. In some cases we have actually kept patients on antibiotics until some
49degree of fusion and spine stability has been achieved and the hardware can be removed.
Functional deficits must be considered in these cases when muscle flaps are used. In particular, the latissimus muscle may be important in using crutches or a wheelchair, and this fact argues against its use in myelomeningocele patients as well as spinal patients with lower extremity weakness. The gluteus muscle is used commonly in paraplegic patients for coverage of the lower lumbar spine and sacrum but may cause a problem with ascending stairs in an ambulating patient.
Suggested Reading
1.Casas LA, Lewis Jr VL. A reliable approach to the closure of large acquired midline defects of the back. Plast Reconstr Surg 1989; 84(4):632.
2.Dumanian GA, Ondra SL, Liu J et al. Muscle flap salvage of spine wounds with soft tissue defects or infection. Spine 2003; 28(11):1203.
3.Foster RD, Anthony JP, Mathes SJ et al. Flap selection as a determinant of success in pressure sore coverage. Arch Surg 1997; 132(8):868.
4.Hochberg J, Ardenghy M, Yuen J et al. Muscle and musculocutaneous flap coverage of exposed spinal fusion devices. Plast Reconstr Surg 1998; 102(2):385.
5.Manstein ME, Manstein CH, Manstein G. Paraspinous muscle flaps. Ann Plast Surg 1998; 40(5) :458.
6.Mathes SJ, Stevenson TR. Reconstruction of posterior neck and skull with vertical trapezius musculocutaneous flap. Am J Surg 1988; 156(4) :248.
7.Ramasastry SS, Schlechter B, Cohen M. Reconstruction of posterior trunk defects. Clin Plast Surg 1995; 22(1):167.
8.Stahl RS, Burstein FD, Lieponis JV et al. Extensive wounds of the spine: A comprehensive approach to debridement and reconstruction. Plast Reconstr Surg 1990; 85(5):747.

Chapter 50
Abdominal Wall Defects
Mark Sisco and Gregory A. Dumanian
Background
Acquired abdominal wall defects can occur as the result of postoperative wound complications, trauma, or surgical resection. Several options exist to manage each of these scenarios. Congenital defects comprise a distinct subset of abdominal wall defects; their treatment is the purview of pediatric surgeons and is not covered here. The objectives of abdominal wall reconstruction, in order of priority, are protection of the abdominal contents, the restoration of visceral support and the production of a natural body contour. Knowledge of abdominal wall anatomy as it relates to the defect is paramount in planning appropriate management. Reconstruction of the myofascial layer restores visceral support and structural stability. Reconstruction of the cutaneous layer provides wound closure and provides an aesthetic outcome.
Preoperative Considerations
Relative vs. Absolute Defects
The plastic surgeon is typically consulted when closure of an abdominal defect cannot be accomplished primarily. Relative defects arise when an increase in the size of the viscera or contraction of the soft tissues of the abdominal wall precludes wound closure. Absolute defects result from loss of soft tissues, as in trauma, necrotizing infection, or tumor resection. Absolute defects are more difficult to treat and are more likely to require flap mobilization.
Layers of the Abdominal Wall
Defects involving the abdominal wall may affect the skin and subcutaneous tissue or the myofascia. A defect of the skin and subcutaneous tissue leads to an open wound. A myofascial defect leads to loss of structural support for the viscera. Finding a suitable surgical solution depends on an analysis of which component is missing and the most straightforward means of replacement or repair.
Location the Defect
Several classifications of abdominal wall defects have been proposed. Mathes et al have devised a classification of abdominal wall defects based on location.
Midline defects with a myofascial deficiency, such as incisional hernias, are usually amenable to meshes, grafts, or the components separation technique, which uses advancement of adjacent myofascial tissue.
Lateral defects, often due to extirpation or trauma, are best treated with local and distant flaps based on the size of the defect.
Practical Plastic Surgery, edited by Zol B. Kryger and Mark Sisco. ©2007 Landes Bioscience.

310 |
Practical Plastic Surgery |
Timing of Wound Closure
Emergent Wound Closure
In critically-ill patients such as those who have undergone damage-control laparotomies for trauma, reconstruction of the abdominal wall may become an issue when edematous intraabdominal contents prohibit wound closure. In these cases, the priority is to restore the protective function of the abdominal wall such that the viscera are protected from dessication and evisceration. Temporary closure methods are suitable because of the likelihood that such patients may need to be reexplored and because prompt resolution of the edema is the rule. Such efforts are usually performed by the trauma surgeon. These methods include sewing nonabsorbable materials, such as IV bags, into the defect and using various improvised and commercial vacuum devices.
Urgent Wound Closure
In unstable patients or in those with acute wound failure complicated by gross contamination or bacterial infection, urgent wound closure is indicated. The primary goal in such situations is to prevent evisceration. Large defects may be simply and quickly closed with absorbable mesh which is then covered with saline-soaked dressings. Definitive reconstruction in such cases is delayed and performed as a second-stage operation. In stable patients, definitive reconstruction with autologous
50 tissue may be considered at that time.
Definitive Wound Closure
When possible, definitive reconstruction should be performed in a single stage. Definitive closure may also be performed following procedures such as those described above, when the patient is stable and the wound is more amenable to reconstructive intervention. When possible, most authors suggest a delay of at least six months following the initial surgery to reduce the likelihood of iatrogenic bowel injury. The key to effective reconstruction of the abdominal wound is tension-free closure at the healing edge. Primary closure, mesh and graft reconstruction, component separation, locoregional flap reconstruction and distant flap reconstruction represent four approaches to the increasingly complex abdominal wound.
Simple Skin Defects
Simple skin defects, when small, may be closed with local skin elevation and primary closure. Larger skin defects can be treated with a negative pressure wound therapy (NPWT) device, skin grafts, local skin flaps, or fasciocutaneous flaps. Local skin flaps include the groin flap, superficial inferior epigastric artery flap, iliolumbar flap, or thoracoepigastric flap. Alternatively, tissue may be expanded while a skin graft or NPWT device is in place.
Musculofascial Defects
Musculofascial defects with intact skin coverage are synonymous with ventral hernias. Primary repair may be attempted for small (<3 cm) defects; however this may be associated with a high recurrence rate. Medium and large defects should be reconstructed with grafts that have sufficient tensile strength to restore the structural integrity of the abdominal wall. They are treated with prosthetic materials or autologous tissue such as fascial grafts, local flaps, or advancement flaps.

Abdominal Wall Defects |
311 |
Open Wounds
Open wounds with exposure of the viscera can be closed when possible by elevating and closing the skin, which allows for subsequent repair as a myofascial defect. When primary closure of the skin is impossible, patients can be reconstructed immediately using local flaps in combination with skin grafts, distant flaps, combinations of prosthetic materials and flaps, or free tissue transfer. Unstable patients with large open wounds can be managed by placing an absorbable mesh and dressing changes.
Mesh and Graft Reconstruction
Mesh is often used for the repair of ventral hernias in which a pure fascial deficit exists. A variety of synthetic materials have been used to reconstruct the fascia successfully. Other materials such as acellular dermal matrix (AlloDerm®) and sodium hyaluronate and carboxymethylcellulose coated mesh (Sepramesh®) are also in early clinical use.
Permanent mesh is most commonly used to close the abdomen. Since there are risks of infection, extrusion and fistula, permanent meshes should be used in clean wounds in which there is enough soft tissue to provide wound coverage. Permanent meshes include polypropylene, polytetrafluoroethylene (PTFE) and polyester. Polypropylene (Prolene®, Marlex®). It has excellent strength at the suture line but may be associated with adhesions. PTFE (Gore-Tex®) demonstrates less in-growth
of surrounding tissue than polypropylene, which confers a theoretical increased risk 50 of hernia recurrence since there is less strength at the suture line. Polyester mesh (Mersilene®) is strong and pliable. It causes a significant inflammatory response in surrounding tissues.
As described above, polyglactin (Vicryl®, Dexon®) absorbable meshes can be used when urgent wound closure is required and in the settings of wound contamination/sepsis or bowel fistula. This mesh provides a base for granulation tissue formation and is retained until infection and critical illness have resolved. The intestines adhere to each other and to the fascial edges, becoming “frozen.” The mesh is then removed and skin grafts are placed. The use of absorbable mesh virtually guarantees the formation of a large ventral hernia and requires second-stage reconstruction, usually six months later.
Nonvascularized fascial grafts have excellent incorporation. As autologous tissue, they are better suited to contaminated wounds than permanent mesh. However, fascial grafts have less tensile strength than PTFE or polypropylene and require creation of a donor defect. They are associated with a significant rate of dehiscence and recurrence.
Flap Reconstruction
Locoregional flaps can replace both the fascial and cutaneous components of the abdominal wall in large open wounds. The rectus abdominis muscle flap is the most commonly used flap in abdominal wall reconstruction. It can be used with or without a skin paddle to reconstruct any area of the abdominal wall, especially in the middle and lower thirds. It can be based on the superior epigastric or the deep inferior epigastric arteries. The tensor fascia lata (TFL) myocutaneous flap, based on the lateral circumflex artery, is useful to reconstruct defects inferior to the umbilicus. The rectus femoris can be used to cover defects involving the lower quadrants and the umbilical and the epigastric areas. Table 50.1 summarizes the local and distant flaps commonly used for abdominal wall reconstruction.
