
Practical Plastic Surgery
.pdf
292 |
Practical Plastic Surgery |
Applying similar techniques, the contralateral breast undergoes the same procedure. Once both breasts have been reduced, temporary sutures or staples are used to approximate the skin and the patient is placed in an upright position. Careful attention is paid to the symmetry of the breast and position of the nipple. Final judgments and adjustments are made, and the patient is placed back in the supine position. If not performed previously, the curvilinear keyhole incision creating the opening for the nipple areolar complex is completed. A buried 2-0 vicryl suture is placed to bring together the junction of the “T”. Some surgeons will place a 10 mm Jackson-Pratt (JP) drain in each breast prior to closure. The skin incisions are closed in layers using interrupted buried 2-0 vicryl deep, 3-0 monocryl in the dermal layer and a subcuticular running 3-0 or 4-0 monocryl or prolene. Steri-strips are applied to the incision lines, followed by sterile 4x8 fluffs or Telfa/Tegaderm and a sports bra.
Vertical Scar/Medial Pedicle
Although it is entitled a medial pedicle, the pedicle is often superomedially-based and full-thickness down to just above the pectoralis fascia. The flap is designed so
46that one-half to one-third of the flap is in the areolar opening and remainder is along the vertical skin incision line. The pedicle width should be equal to the pedicle length, which is usually about 8 cm. The pedicle is de-epithelialized including a rim around the areola. The pedicle is beveled superiorly to create a platform for the nipple-areolar complex and left attached to the chest wall.
The parenchymal breast tissue and skin to be removed are excised in one piece. The tissue laterally is beveled out extensively while there should be minimal beveling in the inferior-medial pole. Dissection continues with undermining down to the inframammary fold. Removing the inferiorly-based, subcutaneous tissue can reduce skin puckering on closure. If the inferiorly-based, subcutaneous tissue is not removed, puckering of the skin will occur. The tissue at the inframammary fold must be excised. Little to no breast tissue should be excised from the superior pole. All tissue is weighed together from each breast and sent to pathology for evaluation. The pedicle is rotated up into position, and a suture is placed at the base of the areola. The inferior border of the pedicle becomes the medial pillar, and this is sutured to the lateral pillar, starting at the base and progressing superficially. Skin closure and dressings are performed as described above.
Free Nipple Graft
An incision is made around the diameter of the areola and the nipple-areola is harvested as a full-thickness graft approximately 40 mm in diameter. The inframammary fold incision is made and the breast tissue is dissected off of the fascia to the level of the nipple. The horizontal limb incisions are made, beveling superiorly, and the inferior breast tissue is removed. An additional wedge of breast tissue is removed from the deep lateral segment of the breast to allow the breast to narrow during closure. The medial and lateral flaps are brought down to the inframammary fold and stapled in place. Excess skin can be removed. The patient is placed in the sitting position and the new nipple position is determined. The patient is lowered to a supine position and the skin around the site of the new nipple-areola is de-epithelialized. The free nipple graft is sutured in place with an absorbable suture and bolstered in place. All tissue is weighed together from each breast and sent to pathology for evaluation.
Applying similar techniques, the contralateral breast undergoes the same procedure. Once both breasts have been reduced, temporary sutures or staples are

Reduction Mammaplasty |
293 |
used to approximate the skin, and the patient is placed in an upright position. Careful attention is paid to the symmetry of the breast and position of the nipple. Final judgments and adjustments are made, and the patient is placed back in the supine position. Skin closure and dressings are performed as described above.
Postoperative Considerations
The patient may remain in the hospital overnight or be discharged the same day. Pain control is managed with oral narcotics. If JP drains are used, they are removed when the drainage drops below 30 ml/day. Many surgeons will leave a patient on oral antibiotics (Keflex) for a few days postoperatively. If drains are used, they may continue the oral antibiotics until the drains are removed. The sports bra should be worn day and night for 8 weeks. Patients should avoid heavy lifting and strenuous activity for 1 month after their surgery. A new baseline mammogram should be performed approximately 6 months postoperatively in all patients over the age of 35.
Pearls and Pitfalls
Markings of the breast meridian, medial and lateral extent of excision, etc., must 46 be individualized to each breast since patients commonly have asymmetry. The preoperative discussion with the patient is essential. She should clearly understand all of
the risks, benefits and alternatives, incisions, scars, etc. prior to arriving in the operating room. Breast evaluation with mammography and physical exam needs to be performed preoperatively, and all breast tissue should be sent to pathology for evaluation.
In dissection of either the inferior pedicle or medial pedicle, the pectoral fascia should be left intact. This will decrease postoperative pain. For the Wise pattern and even the vertical pattern, the final areolar placement can be made after the skin and parenchymal excision by sitting the patient up and examining symmetry. Nipple-areolar symmetry is extremely important in the overall aesthetic outcome.
In the inferior pedicle technique, the medial pocket should be left fuller since this gives a more aesthetic postoperative result. Maintaining a wide pedicle base and the attachments to the chest wall is essential for maintaining vascularity of the nipple-areola complex.
In the medial pedicle design, maintain a wide base of attachment of the entire pedicle to the chest wall. The excision of parenchyma should be greatest in the inferior and lateral pockets. Excision superiorly should only be enough to rotate the pedicle into its new position. Pillar suture placement helps to shape the breast and lift it superiorly. Do not be afraid to reopen incisions several times to remove more tissue for symmetry purposes and to obtain meticulous hemostasis.
Suggested Reading
1.Bostwick IIIrd J. Reduction mammaplasty. Plastic and reconstructive breast surgery. 2nd ed. St. Louis: Quality Medical Pub, 2000:371-498.
2.Chadbourne EB, Zhang S et al. Clinical outcomes in reduction mammaplasty: a systematic review and meta-analysis of published studies. Mayo Clinic Proceedings 2001; 76(5):503-510.
3.Cohen BE, Ciaravino ME. Reduction mammaplasty. In: Evans GRD, ed. Operative Plastic Surgery. New York: McGraw-Hill Professional (Appleton and Lange), 2000:613-630.
4.Cruz-Korchin N, Korchin L. Breast-feeding after vertical mammaplasty with medial pedicle. Plast Reconstr Surg 2004; 114(4):890-4.
5.Hall-Findlay EJ. A simplified vertical reduction mammaplasty: Shortening the learning curve. Plast Reconstr Surg. 1999; 104(3):748-759, (discussion 760-763).
6.Hall-Findlay EJ. Vertical breast reduction. Seminars Plast Surg 2004; 18(3):211-224.

Chapter 47
Sternal Wounds
Jonathan L. Le and William Y. Hoffman
Incidence
The majority of sternal wounds seen by the plastic surgeon are infections secondary to dehiscence of a median sternotomy following a postoperative infection. The incidence of median sternotomy dehiscence is reported in up to 5 percent of open heart procedures. The patients at highest risk are diabetics undergoing coronary revascularization using the internal mammary artery since this procedure results in impaired blood supply to the healing sternal incision.
Initial attempts to manage this complication conservatively with open drainage, debridement, and packing often resulted in bypass graft exposure, desiccation of wound margins, osteomyelitis and even death. Mortality due to sternotomy wound dehiscence is higher in patients with septicemia, perioperative myocardial infarction, or those requiring an intraaortic balloon pump. Mortality rates previously reached 50 percent; however, the development of wound reconstruction techniques using wide debridement and muscle or musculocutaneous flap transposition has reduced mortality rates to less than 5%.
Wound Classification
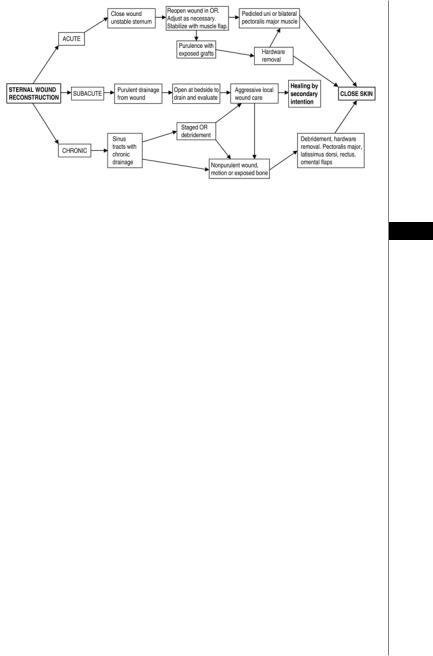
Pairolero’s classification scheme identifies three types of sternal wound infections:
•Type I (acute) infected sternotomy wounds occur one to three days postoperatively and present with serosanguinous drainage. Wound cultures are negative and cellulitis, costochondritis and osteomyelitis are absent. These wounds require reexploration, minimal debridement and rewiring of the sternum. Reconstructive surgeons are typically not consulted.
•Type II (subacute) wounds present two to three weeks postoperatively and involve purulent mediastinitis with positive wound cultures, cellulitis, costochondritis and osteomyelitis.
•Type III (chronic) infected sternotomy wounds occur months to years after cardiac procedures and display draining sinus tracts as a result of chronic
costochondritis and osteomyelitis.
Type II and III wounds require thorough debridement and may depend on temporary open-wound dressing changes for adequate infection control. Figure 47.1 provides an algorithm for the most appropriate management based on the presentation of the wound.
The majority of patients with postoperative mediastinitis have monomicrobial infections although virtually any organism can be responsible. Approximately 50 percent of infections are due to Gram positive bacteria, such as Staphylococcus aureus, with the remainder due to Gram negative bacilli, such as Pseudomonas
Practical Plastic Surgery, edited by Zol B. Kryger and Mark Sisco. ©2007 Landes Bioscience.
Sternal Wounds |
295 |
|
|
|
|
|
|
|
Figure 47.1. An algorithm for management of sternal wounds.
aeruginosa. Initial empiric antibiotic therapy should consist of broad coverage 47 against these organisms. Rarely, postoperative mediastinitis is due to unusual organisms such as fungi, Legionella, or Mycoplasma hominis.
Preoperative Evaluation
Careful examination of the patient with a sternotomy wound dehiscence is extremely important. The primary contraindication to reconstructive surgery is a wound with active purulence. Such a wound requires thorough debridement prior to flap coverage to avoid possible flap failure. Patients who are hemodynamically unstable, have poor pulmonary or cardiac reserve, or are terminally ill should not be considered for surgery.
Appropriate laboratory and radiographic studies must be performed prior to operating on patients with infected sternotomy wounds. Easily accessible fluid collections should be aspirated and sent for culture. If the patient clinically deteriorates or further signs of wound breakdown are observed (such as increased erythema, drainage, dehiscence, or systemic signs of infection) then wound cultures should be obtained and sent. Wound cultures should include a quantitative microbiology count, a tissue specimen for analysis and sternum biopsies for culture.
Plain chest radiographs should be examined to evaluate the condition of the lung fields, to check for the presence of effusions, and to determine the number, position and condition of the sternotomy wires. Computed tomographic scans are usually not helpful unless the patient has an elevated fever and white blood cell count and the previously opened wound does not correspond with the illness. The assessment of possible osteomyelitis using a bone scan may have limited value for the acute wound due to the presence of inflammation and tracer uptake. Diagnosis of osteomyelitis is most accurate when bone biopsies are obtained rather than relying solely on clinical information.
Operative Approaches
Numerous surgical options are available for closure: the unilateral pectoralis major muscle turnover flap, the unipedicle pectoralis major muscle rotation advancement flap, bilateral myocutaneous pectoralis major muscle flaps, rectus

296 |
Practical Plastic Surgery |
abdominus muscle flap, latissimus dorsi muscle flap, omental flap, microsurgical free flap and vacuum-assisted closure. Although skin flaps were used historically, vascularized regional muscle and muscluocutaneous transposition flaps are now preferred due to their greater blood flow, obliteration of dead space and faster healing time from quicker resolution of infection. Omental and microvascular free tissue transfer are reserved for situations when no local muscle flaps are available or when other alternatives have failed.
Pectoralis Major Flap
The pectoralis major muscle is a common choice for coverage of a median sternotomy wound because of the proximity of the donor tissue, relative ease of harvest, versatility and its wide arc of rotation. The muscle originates on the clavicle, sternum, six upper ribs and aponeurosis of the external oblique muscle and inserts on the intertubercular sulcus of the humerus. This flap may be based on either of its two vascular pedicles, the thoracoacromial artery or the segmental perforators from the internal thoracic artery (see Appendix I).
47 Pectoralis Turnover Flap
By basing the flap medially on the segmental parasternal perforators from the internal thoracic artery, the muscle can be turned over on itself. The muscle is dissected from the chest wall lateral to medial to avoid injury to the perforators. The thoracoacromial pedicle is ligated, and the humeral end of the muscle is disinserted and placed in the sternal defect. The limitations of this approach are that one wastes part of the flap to allow the muscle to turnover and possibly creates a raised contour deformity of the anterior chest wall that would put tension on the skin closure.
Pectoralis Transposition/Advancement Flap
When the internal thoracic arteries are sacrificed bilaterally, the flap can be based on the thoracoacromial pedicle alone. The muscle is dissected off the sternum, disinserted from the humerus and advanced into the defect. This technique affords greater muscle mass to fill upper mediastinal dead space but without a wide arc of rotation the inferior aspect of the wound may remain inadequately covered. Wide undermining of skin flaps provides easier skin closure.
Bilateral myocutaneous medial advancement of both pectoralis major flaps may be considered for coverage of large defects. The flaps are elevated in the relatively avascular plane just deep to the pectoralis major muscles. This approach is best used in the absence of purulent mediastinitis as it does not fill the mediastinal dead space.
Rectus Abdominus Flap
The rectus is effective for covering inferior sternal defects and is used to supplement a pectoralis flap or when pectoralis muscle is unavailable. It may be used as a turnover flap based on the superior epigastric artery (see Appendix I). If only one internal thoracic artery is available, the flap may need to be harvested from the contralateral side. If both internal thoracic arteries have been sacrificed, then the flap can be based on the eighth anterior intercostal perforator to the rectus muscle; however, the distal third of the rectus muscle may become ischemic using this approach.
The rectus originates at the pubic crest and inserts at the xiphoid and fifth, sixth and seventh costal cartilages. The rectus muscle is harvested through a midline or

Sternal Wounds |
297 |
paramedian skin incision. After the anterior rectus sheath is incised the muscle is divided at the appropriate level and elevated from the posterior rectus sheath towards the sternum. Once adequate mobilization has been achieved, the flap is transposed into the wound.
Latissimus Dorsi Flap
This flap is best used for smaller defects and should be reserved as a salvage flap in the event of pectoralis muscle flap failure. The muscle originates at the posterior iliac crest and spinous processes L7 to S1 and inserts at the crest of the lesser tubercle of the humerus. It is based on its major pedicle, the thoracodorsal artery, when it used for sternal defects (see Appendix I). When adequately mobilized, it can extend over the anterior chest to cover the mediastinum.
Omental Pedicle Flap
The broad, pliable, fatty nature of the omentum allows it to fill the deep spaces of large wounds. In addition the flap has a generous source of lymphatics and immune cells that support the clearance of infection. It can provide reliable coverage
for deep sternal infections such as osteomyelitis, costochondritis, or mediastinitis. 47 Its blood supply can be based on either the right or left gastroepiploic artery.
A midline laparotomy is the usual approach followed by lysis of adhesions from any previous abdominal surgery. After the pedicle from either the left or right gastroepiploic artery is identified, added mobilization may be achieved by dividing the short gastric vessels along the greater curvature of the stomach. This should be done cautiously as gastric outlet obstruction is associated with excessive cranial traction on the antrum of the stomach during mobilization and flap inset. Omental flaps heal without intraabdominal problems or chest wall instability 95% of the time. Complications include ventral hernia formation, wound infection and bowel injury. Laparoscopic harvest of the omentum is well described in the literature and should be considered for patients who are poor candidates for a laparotomy.
Microvascular Free Flaps
Free tissue transfer is usually reserved for conditions where local flaps are unavailable or have previously failed. Contralateral latissimus dorsi or tensor fascia lata are the most popular for this purpose. There are many potential recipient vessel sites for anastomosis of the donor pedicle including the axillary, thoracodorsal, subscapular, or internal mammary artery.
Postoperative Complications
Complications of infected sternal wound reconstruction include hematoma, seroma, dehiscence and sternal necrosis with osteomyelitis.
The formation of a hematoma is infrequent, but this complication generally requires reoperation. The most common cause of hematoma is premature reinstitution of anticoagulation therapy. Careful attention to hemostasis, meticulous pedicle dissection, and closure over suction drains will also help prevent this complication from occurring.
Seroma formation is infrequent but the risk may increase when two muscle flaps are used simultaneously. Closure of flaps over drains is an effective preventative measure. This minimizes dead space and provides for early flap adherence.

298 |
Practical Plastic Surgery |
Dehiscence is more likely to occur in obese patients, older patients with chronic obstructive pulmonary disease, patients on prolonged ventilatory support, diabetics and patients with sepsis. In women with large, pendulous breasts the use of surgical bras and tapes will help prevent distraction on the medial chest and separation of the flaps.
Sternal necrosis and osteomyelitis may occur in patients with profound sepsis, Gram-positive infections, and when debridement is not adequate.The sternum should be debrided back to viable, bleeding bone. Surgery performed in the absence of frank purulence will also help lower the risk of infection recurrence.Reinfected wounds generally heal spontaneously when reopened, since foreign bodies and nonviable tissues have already been removed.The application of dedicated local wound care and/or vacuum-assisted closure therapy usually results in wound closure by secondary intention. Secondary flap reconstruction is only necessary in selected severe cases.
Pearls and Pitfalls
First and foremost, the timely recognition of an infected sternal wound is critical. Despite extensive data verifying the utility of flap closure of these wounds, it is
47not uncommon to see patients with mediastinitis treated with rewiring of the sternum, irrigation-drainage systems or some other alternative. Our experience has been favorable with simultaneous debridement and flap closure of the mediastinal wound, and this is done routinely at our institution unless the patient is medically unstable due to sepsis. Often the left sternum is more affected due to sacrifice of the left IMA for coronary revascularization. In these cases, we have found that debridement of the left side only and preservation of at least a portion of the right is still effective.
The manubrium is often unaffected by the infectious process, probably due to its more proximal blood supply. The xyphoid is often involved in severe cases. If the pectoralis muscles is detached from the clavicle as well as the humerus it can usually reach the xyphoid. Finally, new technology should be noted. There are recent reports that rigid fixation of the sternum can markedly reduce mediastinal infections and even be used in some infected cases. Laparoscopic harvest of omental flaps has also been reported for treatment of sternal wounds and might actually be less morbid than muscle flaps.
Suggested Reading
1.Jurkiewicz MJ et al. Infected median sternotomy wound: Successful treatment by muscle flaps. Ann Surg 1991; 738:1980.
2.Nahai F et al. Primary treatment of the infected sternotomy wound with muscle flaps: A review of 211 consecutive cases. Plast Reconstr Surg 1989; 84:434.
3.Obdeijin MC et al. Vacuum-assisted closure in the treatment of poststernotomy mediastinitis. Ann Thorac Surg 1999; 68:2358.
4.Pairolero PC, Arnold PG, Harris JB. Long-term results of pectoralis major muscle transposition for infected sternotomy wounds. Ann Surg 1991; 213:583.
5.Serry C et al. Sternal wound complications. Management and results. J Thorac Cardiovasc Surg 1980; 80:861.
6.Wening JV et al. Repair of infected defects of the chest wall by transposition of the greater omentum. Br J Clin Pract 1990; 44:311.

Chapter 48
Chest Wall Defects
Jason Pomerantz and William Hoffman
Introduction
As is true for reconstructive surgery in general, successful repair of chest wall defects involves a sequence of steps, each of which must be accomplished in order to proceed appropriately and safely to the next step. The precise nature of the defect will dictate the particular reconstructive requirements and options. With respect to function, chest wall defects may require repair of the skeletal support system in addition to soft tissue coverage in order to restore normal respiratory mechanics.
Preoperative Considerations
The evaluation of a patient requiring chest wall reconstruction begins with an assessment of the overall medical and functional status. Many patients with chest wall defects have significant comorbid conditions. Prior to definitive defect reconstruction, the patient’s neurological, cardiovascular, and respiratory function should be stable and optimized. Definitive wound closure is often deferred until the patient’s operative risk is acceptable, and interim management may include mechanical ventilation, local wound care, debridement and wound VAC placement for partial-thickness defects. With respect to the timing of repair, defects should be repaired as soon as possible after major organ system stabilization in order to decrease the incidence of wound and other related complications. In addition to impact on survival, chest wall defects must be evaluated in terms of their effect on quality of life, taking into account the patient’s functional status, motivation and prognosis.
Chest Wall Defect Analysis
The specific etiology of a chest wall defect will influence the subsequent reconstructive plan. The underlying cause of the defect can be classified into one the following: trauma, tumor, infection, radiation, or congenital. A number of specific considerations arise depending on the etiology of the defect. For example, in any trauma case it is important to fully evaluate concomitant injuries. Reconstructive surgery is performed after life-threatening problems are recognized and addressed. With respect to operative planning, in traumatic wounds it is imperative to consider the “zone of injury” which may extend well beyond apparent borders of viable tissue. Generally, only tissue outside of the zone of injury should be used for wound coverage. Infected or traumatic wounds may require extensive debridement, potentially resulting in a significant alteration of the operative plan. This should be anticipated before beginning the operation.
Irradiated tissue poses additional issues. Previously irradiated tissue is often fibrotic and may also be poorly vascularized. Such tissue is less amenable to mobiliza-
Practical Plastic Surgery, edited by Zol B. Kryger and Mark Sisco. ©2007 Landes Bioscience.

300 |
Practical Plastic Surgery |
tion as opposed to healthy tissue and is generally not a good source of tissue for wound coverage. If future irradiation is planned at the site of the defect, then reconstructive options utilizing bulkier, better vascularized tissue (flaps as opposed to skin grafts) are preferred.
Defect analysis requires an accurate anatomical description of the site. The location of the defect on the chest wall will dictate the reconstructive options. The chest wall can be divided into four regions:
•The anterior chest is located (medial to lateral) between the parasternal line and anterior axillary line and (superior to inferior) between the clavicle and superior costal margin. Breast defects may occur along with chest wall defects in this region, and breast reconstruction should be considered in conjunction with planning of chest wall reconstruction.
•The superior chest is located (medial to lateral) between the base of the neck to acromioclavicular joint and (anterior to posterior) between the deltopectoral groove and the spine of the scapula. In this region, absence of the clavicle does not impair chest wall stability, but exposure of the subclavian vessels and brachial plexus may occur.
•The lateral chest is located (medial to lateral) between the anterior and poste-
48rior axillary lines and (superior to inferior) between the apex of the axilla and inferior costal margin. Wounds in this region may involve exposure of the axillary vessels or the musculocutaneous, median or ulnar nerves.
•The posterior chest is located (medial to lateral) between the posterior midline and the posterior axillary line and (superior to inferior) between the spine of the scapula to the posterior costal margin at L1. Central posterior chest wall defects (spinal wounds) and central anterior chest wall defects (sternal wounds) are dis-
cussed in separate chapters in this text.
After location, chest wall defects are described in terms of depth: partial- or full-thickness. Partial-thickness defects may involve loss of skin and varying amounts of subcutaneous fat and muscle. The wound base may contain muscle or bone. Full-thickness defects involve loss of soft tissue as well as bone. In full-thickness chest wall defects, the size of the bony defect in centimeters, as well as the number of consecutive ribs missing, dictates the requirement for restoration of chest wall stability because of exposure of vital organs as well as the potential for paradoxical chest wall motion during respiration. It is important to note that abnormal chest wall motion may not be obvious during mechanical ventilation, and observation of spontaneous, unassisted respirations is required.
Reconstructive Options
The goal of chest wall defect closure is to perform adequate debridement followed by safe wound coverage that restores form and function. In addition, intrathoracic dead space should be obliterated. Reconstructive options include direct wound closure, healing by secondary intention, skin grafting, local flap options, microsurgical tissue transfer and tissue expansion. Accurate defect analysis permits development of a suitable plan for wound coverage or reconstruction. For partial-thickness wounds, including those devoid of muscle at the wound base, skin grafting is a viable option if there is intact periosteum on any exposed bone. However, skin grafting is not ideal because of the resulting contour deformity and because it generally is more prone to breakdown. In addition, if radiation is planned or possible in the future, a muscle flap is indicated.

Chest Wall Defects |
301 |
If a defect involves the full-thickness of the chest wall, a muscle flap is always indicated, and the decision must be made whether skeletal stability needs to be restored. In addition to exposing the thoracic viscera, defects encompassing at least four consecutive ribs and greater than 5 cm in size are generally thought to have a greater likelihood of involving a flail segment and in these cases skeletal stabilization is indicated. Often, muscle or musculocutaneous flaps will result in a stable chest wall without significant paradoxical motion. Fasciocutaneous or omental flaps do not provide adequate structural support and require simultaneous rib reconstruction. More extensive full-thickness defects require skeletal stabilization with autogenous bone or synthetic materials in addition to soft tissue coverage. Split rib grafts are a good option in elective clean cases. However, given the wealth of available synthetic materials, the most commonly used of which are prolene mesh or composite methyl methacrylate/marlex (used for larger defects), autogenous bone grafts are being used less frequently. Synthetic materials are sutured to intact bony structures on either side of the defect, followed by coverage with a well-vascularized flap.
Muscle flaps commonly used in chest wall reconstruction all have adequate arcs of rotation, major vascular pedicles and adequate bulk. Particular flaps are
chosen based on defect location and size. Table 48.1 lists the commonly used 48 muscle flaps in chest wall reconstruction. It is important to note any prior procedures in the region, (e.g., thoracotomy) that may have sacrificed the pedicle of an otherwise suitable flap. In addition to flap transposition, tissue expansion and microsurgical free flaps are options as well. Tissue expansion offers the advantage
of providing similar skin characteristics and avoids donor site morbidity, but is contraindicated in cases involving chronic infection or radiation injury. If tissue expansion is chosen, the defect may be temporarily covered with a skin graft during the 2-3 month period of expansion. Free flaps are rarely needed for chest wall reconstruction unless there is associated injury to the pedicles of other flaps, and more distant flap transpositions do not provide adequate arcs of rotation. However, with improvement in microsurgical technique and outcomes, free tissue transfers are being used more frequently, especially for the largest and most complex cases. Typical free flaps for chest wall reconstruction include the contralateral latissimus dorsi, tensor fascia lata or rectus abdominis.
A general treatment algorithm for managing chest wall defects is presented in Figure 48.1.
Postoperative Considerations
Suction drains placed during surgery are important for preventing postoperative fluid accumulation at the defect or donor site. Any violation of the thoracic cavity usually requires a chest tube in the immediate postoperative period until there is no evidence of an air leak. Most surgeons continue antibiotic therapy while drains are in place in order to decrease the incidence of infection, especially if synthetic materials such as mesh or methyl methacrylate are used.
Chest wall defects, especially those involving rib resection can be very painful, and aggressive postoperative pain control is important for optimal wound healing. Depending on the location, an epidural may be beneficial. Intercostal blocks may also be useful in addition to the standard narcotics PCA and oral pain medications.
