
Practical Plastic Surgery
.pdf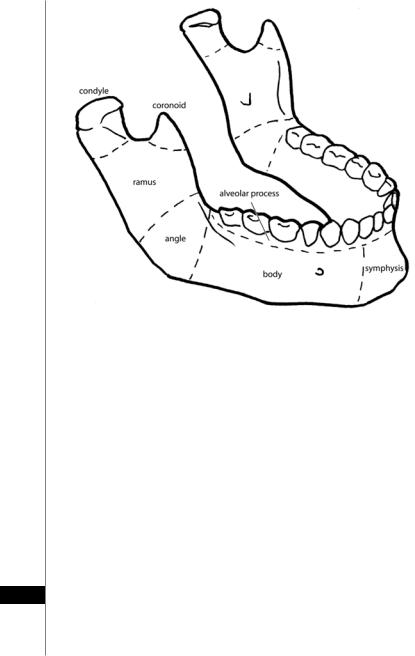
212 |
Practical Plastic Surgery |
||
|
|
|
|
|
|
|
|
Figure 34.1. Anatomy of the mandible.
When mandibular reconstruction is needed after tumor resection, the timing of the reconstruction should consider the tumor characteristics, the amount of tissue to be resected and the possibility of achieving clear margins. If the resection margins are questionable, a two-stage procedure after several months would be more preferable.
Choices for reconstruction include composite free flaps, nonvascularized autologous bone grafts, or synthetic material. Immediate reconstruction with allograft materials or with autogenous bone may be associated with a 15-47% infection rate and loss of the transplant/implant. Therefore, proper preoperative dental evaluation should identify any potential decaying teeth that might serve as a source of infection. Such teeth should be addressed either by restoration or extraction.
Techniques for Bony Reconstruction
Nonvascularized Autogenous Bone Grafts
Bone grafts harvested from the iliac crest, rib, or calvaria are used for nonvascularized bone grafts. Autogenous bone grafts are more resistant to infection than allogenic grafts. They are usually of good quality and provide osteoconductive
34and osteoinductive properties. The rib is used for condylar reconstruction and can be shaped according to the requirements. Iliac bone grafts and calvarial bone grafts can be used to reconstruct alveolar defects or bone defects less than 5 cm. The graft is shaped and its cortex is perforated at multiple sites to enhance vascularity and eventual resorption. While rib grafts have proven ideal for condylar reconstruction, iliac crest grafts are better suited for the maintenance of dental implants.

Mandible Reconstruction |
213 |
The introduction of osteosynthetic plates markedly improved the outcomes of mandibular reconstruction. These plates are relatively easy to apply, provide a firm platform for healing, and are associated with low placement site morbidity. In addition, rigid internal fixation eliminates the need for external or intermaxillary fixation (IMF), maintains the appropriate dental relationships, reduces operative time and can provide effective condylar replacement.
Nonvascularized Alloplastic Bone Grafts
Allografts for mandible reconstruction are mostly comprised of freeze-dried bone. Such bone grafts are only suitable for small defects of the mandible where the continuity of the mandible is intact. The advantages of allografts include the relative ease of availability without a donor defect. The major disadvantage of allograft material is that it is prone to infection and only provides a matrix for osteoconduction. Alloplastic materials are available in paste, powder, or block form which may be easily contoured to fit the required shape. The lack of osteoinductive properties secondary to the absence of vascularity and cellular components limits the use of such material in radiated or poorly vascularized tissue. Significant failure rates (48%) are associated with implantation at radiated mandibular sites, compared to nonirradiated tissues (30%). Allogeneic bone cribs have been called the ideal vehicles for particulate cancellous bone marrow grafts. Bioresorbable and biocompatible, allogeneic cribs are easily adaptable and less prone to the poor graft regeneration seen with the use of alloplastic materials.
Vascularized Bone Grafts
With the development of the modern microsurgical technique, the reconstructive surgeon now has the ability to use a composite free flap for mandibular reconstruction. In many centers, this has become the standard of care for reconstructing large mandibular defects.
Fibula Free Flap
The fibula flap provides a large amount of cortical bone, allowing for multiple osteotomies to achieve the curvature of the mandible. This flap is very versatile and can be harvested as an osteomyocutaneous flap by incorporating a portion of the soleus muscle to provide additional soft tissue bulk. The vascular pedicle is the peroneal artery and vein (up to 8 cm length) and a skin island measuring approximately 10 × 30 cm. The free fibular graft provides up to 24 cm in length of bony material. There usually is sufficient bone height in the newly reconstructed mandible for future placement of a dental prosthesis. This flap provides osteocutaneous coverage that is reliable, durable and aesthetically acceptable to most patients. Disadvantages include poor donor site cosmesis, the limited amount of cutaneous tissue and a potential donor site neurapraxia. A detailed discussion of the design and harvest of this flap is provided in Appendix I.
Iliac Crest Free Flap
The iliac crest flap provides a curved, cortical section of bone that can be used to reconstruct the mandibular symphysis and the curved body region; 10-14 cm of 34 corticocancellous bone can be harvested. The vascular pedicle consists of the deep circumflex iliac artery and vein providing up to 6 cm of length. The soft tissue island
can measure as large as 16 cm in length, and it can be harvested with the internal oblique muscle. Drawbacks may include donor site morbidity, abdominal wall weak-

214 |
Practical Plastic Surgery |
ness or herniation, potential injury to the lateral femoral cutaneous nerve, and delayed postoperative ambulation.
Radial Forearm Free Flap
The radial forearm free flap is used mostly for soft tissue coverage, for example, in reconstruction of the anterior floor of the mouth after a cancer resection. This flap has limited use in mandibular reconstruction due to the limited length of radius that can be harvested, as well as the relatively short height of the harvested bone. It is based on the radial artery and venae comitantes in association with the cephalic vein. The pedicle can measure up to 20 cm in length, making it very versatile for use in sites distant from the recipient vessels. The skin paddle can be harvested as a sensate skin island receiving innervation from the lateral antebrachial cutaneous nerve (C5-C6). Disadvantages in using this flap include a visible donor site with potential skin graft loss over the flexor tendons and potential intra-oral hair growth. The nondominant forearm should be used, and adequate ulnar artery flow in the hand should be verified prior to the procedure. Preoperative laser hair removal should also be considered. A detailed discussion of the harvest of this flap is provided in Appendix I.
Temporomandibular Joint (TMJ) Reconstruction
The TMJ region poses unique reconstructive challenges. Historically TMJ reconstruction has been plagued with failed attempts using prosthetic joint devices. Recently, an implantable titanium glenoid fossa and/or condylar prostheses have been used at many institutions; however long term outcomes are still unknown. Whenever feasible, autogenous rib reconstruction in this region provides the best form and function. Costochondral grafts consisting of rib and associated cartilage allow for the tissue remodeling in response to stress and can provide good functional results despite the lack of an articular disc. Futhermore, in the pediatric patient the rib graft will continue to grow along with facial skeleton. Another reconstructive option is an extended free fibula flap with soft tissue arthroplasty. This flap uses a periosteal sleeve which improves subsequent TMJ reconstruction.
Distraction Osteogenesis
Mandibular defects of more than 5 cm are best reconstructed with an autologous vascularized flap. For smaller defects, several centers have studied the use of distraction osteogenesis (DO) as an alternative procedure. DO is probably best suited for small segmental defects. Recent reports describe success with DO in replacing defects up to 5 cm in the angle, body, and symphyseal regions. DO has an excellent track record in the treatment of craniofacial anomalies involving mandibular insufficiency.
The process of DO involves the gradual distraction of the bony segments flanking the defect, and the synthesis of new bone within the gap. The younger the patient, the less distraction time is required. Adults require a slightly longer period of distraction because their bone regenerative capabilities are slower than those of adolescents or infants. The advantages of DO are that it eliminates the
34need for bone grafts and the morbidity of donor sites. The entire procedure can usually be done intraorally without additional facial incisions. When adequate healing has been achieved, the device can be removed in a short, office-based procedure. In summary, DO is best suited for cases involving small defects, and proper patient selection is important.

Mandible Reconstruction |
215 |
Pearls and Pitfalls
Tissue Engineering
The future of mandibular reconstruction is headed the way of tissue engineering. In 2004, the first significant human mandibular replacement with engineered osseous tissue was described in a patient following subtotal mandibulectomy resulting in a 7 cm defect. After the computer-aided production of a titanium cage customized to the patient’s defect, the structure was filled with bone mineral blocks and infiltrated with a mixture of recombinant human bone morphogenetic protein and autologous bone marrow. The transplant was implanted into the latissimus dorsi muscle. Following 7 weeks of in vivo maturation, a free bone-muscle flap based on the thoracodorsal vasculature was transplanted into the mandibular defect. Skeletal scintigraphy demonstrated bone remodeling and mineralization within the graft both before and after transplantation. Clinically, both aesthetic form as well as excellent functional capacity was regained with no unforeseen complication. This novel graft is expected to accommodate eventual dental implantation, and potentially the removal of the titanium scaffold. Currently, stem cell research is advancing in manner that eventually may eliminate all of the aforementioned therapies. We anticipate that the cutting edge research in stem cell biology will eventually lead to human trials and ultimately a reliable technique for providing soft and hard tissue replacement.
Suggested Reading
1.Coleman IIIrd JJ. Mandible reconstruction. Oper Tech Plast Reconstr Surg 1996; 3(4):213.
2.Herford AS, Ellis E. Use of a locking reconstruction bone plate/screw system for mandibular surgery. J Oral Maxillofac Surg 1998; 56:1261.
3.Hidalgo DA. Aesthetic improvements in free-flap mandible reconstruction. Plast Reconstr Surg 1989; 84:71.
4.Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst 1988; 48:1.
5.Mathes S, Nahai F. Reconstructive surgery principles, anatomy, and techniques. New York: Churchill Livingstone, 1997:1353-1370.
6.Roumanas ED, Markowitz BL, Lorant JA. Reconstructed mandibular defects: Fibula free flaps and osseointegrated implants. Plast Reconstr Surg 1996; 99:346.
7.Swanson E, Boyd JB, Manktelow RT. The radial forearm flap: Reconstructive applications and donor-site defects in 35 consecutive patients. Plast Reconstr Surg 1990; 85:258.
8.Taylor GI. Reconstruction of the mandible with free iliac bone grafts. Ann Plast Surg 1986; 9:361.
9.Terheyden H, Behrens E. Growth and transplantation of a custom vascularised bone graft: In a man. Lancet 2004; 364:766.
10.Weinzweig N, Weinzweig J. Current concepts in mandibular reconstruction by microsurgical free flaps. Surg Technol Int 1997; VI:338.
34

Chapter 35
The Facial Nerve and Facial Reanimation
Zol B. Kryger
Relationship with the Superficial Musculoaponeurotic System (SMAS)
An understanding of the SMAS is critical in order to avoid injuring the facial nerve during facial surgery. The SMAS is a fascial layer that attaches the facial muscles to the overlying dermis. In the lower face, the SMAS is continuous with the platysma. Superiorly, the SMAS extends to the level of the zygomatic arch where its fibers are anchored. Above the arch, the temporoparietal fascia is the equivalent of the SMAS. It joins the deep temporal fascia which extends into the scalp region.
In the lower face and neck, the facial nerve runs deep to the platysma and the SMAS. Toward the midline, the nerve continues deep to the SMAS and runs superficial to the masseter muscle. In the midface and cheek region, the nerve runs within the SMAS, but as it passes over the zygomatic arch, it becomes more superficial. In the temporal region, the facial nerve travels within the temporoparietal fascia. Thus, it becomes apparent why facelifts are performed in the subcutaneous or sub-SMAS planes.
Course of the Extracranial Facial Nerve
The facial nerve enters the face upon exiting the stylomastoid foramen. At this point it is purely a motor nerve. The nerve then travels 15-20 mm sandwiched between the digastric and stylohyoid muscles before entering the parotid gland. Prior to its entrance into the parotid, it sends off a small branch to the posterior digastric and stylohyoid muscles. It also gives off the posterior auricular nerve which travels to the posterior auricular and occipitalis muscles.
Locating the Main Trunk
During total parotidectomy, it is essential to locate the facial nerve. This is done by mobilizing the parotid superiorly and the sternocleidomastoid laterally, which will reveal the posterior belly of the digastric. A key landmark in identifying the main trunk is the cartilaginous tragal pointer. It is located by following the posterior belly of the digastric towards its insertion at the mastoid, and releasing the parotid attachment to the cartilage of the external auditory canal. The main trunk lies about 1 cm deep and slightly inferior and medial to the tragal pointer. A branch of the occipital artery often can be found in close proximity, lateral to the nerve.
Intra-Parotid Anatomy
Within the substance of the parotid, the facial nerve travels in a fibrous plane between the deep and superficial lobes. At the pes anserinus, it divides into two major divisions. One division travels superiorly and the other inferiorly. These major divisions become five branches that exit the parotid: temporal (frontal), zygomatic, buccal, marginal mandibular and cervical. The buccal and zygmotic branches have a
Practical Plastic Surgery, edited by Zol B. Kryger and Mark Sisco. ©2007 Landes Bioscience.

The Facial Nerve and Facial Reanimation |
217 |
number of interconnections; however the temporal and marginal mandibular branches are usually terminal endings that don’t arborize with other branches. In addition, the temporal and marginal mandibular branches are at the greatest risk of 35 injury during surgery. The incidence of injury during rhytidectomy is less than 1%.
Temporal Branches
The temporal branches run within the SMAS up to the level of the zygomatic arch. Cranial to this point the temporal (frontal) branch becomes more superficial and enters the temporal region within the temporopariatel fascia. The frontal branch runs roughly along an upward sloping line extending from 5 mm below the tragus to 15 mm above the lateral aspect of the eyebrow as described by Pitanguy et al. After crossing the zygomatic arch, the frontal branch travels in the superficial layer of the deep temporal fascia (temporopariatel fascia). It penetrates the undersurface of the frontalis muscle. To avoid injuring the nerve during elevation of the facial flap, one should dissect in the subcutaneous plane (superficial to the frontal branch) or in the sub-SMAS, “deep” plane (deep to the nerve).
Marginal Mandibular Branches
These branches originate from the mandibular division that runs along the inferior border of the body of the mandible in 80% of cases, or within 1-2 cm below the mandible in the remaining cases. These branches run deep to the platysma and course more superficially about 2 cm laterally to the corner of the mouth.
In children the facial nerve anatomy is not as predictable as in the adult, and the described landmarks may not be accurate. In fact, the facial nerve may be located in a more superficial plane then in adults.
Muscles of Facial Expression
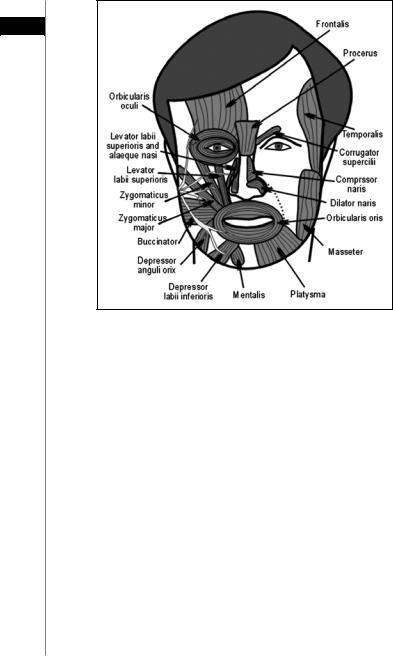
There are 17 main paired muscle groups in the face (Fig. 35.1). The facial nerve innervates these muscles from their deep surface, with the exception of three muscles: the buccinator, levator anguli oris and mentalis. The important muscles of facial expression, their function and their innervating branches are shown in Table 35.1.
Reanimation of the Paralyzed Face
Causes of Facial Paralysis
The etiologies of facial paralysis are quite varied. Intracranial causes include congenital abnormalities, malignancies, degenerative diseases, trauma, vascular conditions and other rare causes. Intratemporal causes include malignancy, trauma, infections, Bell’s palsy, osteopetrosis and iatrogenic causes. Extracranial causes include malignancies (parotid gland as well as tumors of adjacent structures), trauma and iatrogenic injury. Bell’s palsy, or idiopathic facial nerve palsy, is the most common cause of facial nerve paralysis even though 85% of people with Bell’s palsy will recover spontaneously.
Several important physical finding can help to distinguish intracranial (upper motor neuron) from extracranial causes of facial paralysis. Both supranuclear areas provide contributions to the frontal and upper orbicularis occuli muscles. Therefore, these muscles may be partially spared if the etiology is intracranial, creating an ipsilateral “lower face” paralysis. In addition, during periods of intense emotion, facial movements may appear on the affected side. It is important to remember that the extracranial facial nerve is a purely motor nerve; therefore extracranial paralysis
218 |
Practical Plastic Surgery |
35
Figure 35.1. The major muscles of the face. Reprinted with permission from Microsurgeon.org.
should not involve decreased lacrimation (superficial petrosal nerve), changes in hearing (nerve to stapedius) or changes in taste (chorda tympani).
Preoperative Planning
It is essential to evaluate the patient carefully, in order to determine the cause and extent of paralysis and the status of the muscles involved. A history is obtained, focusing on the onset and duration of weakness. A complete physical exam of the head and neck including a cranial nerve exam is performed. The muscles of facial expression are evaluated for bulk, symmetry and function—both statically and dynamically. In addition, electrical testing is performed to determine the physiologic status of the facial nerve branches and the muscles of the face. Such tests, however, are not entirely accurate and tend to overestimate the extent of functional loss. High resolution helical CT is of value in localizing the precise site of pathology. A number of grading schemes for facial nerve paralysis have been described. The House-Brackmann Grading System is most widely used:
Grade Description
INormal facial muscle function
II |
Mild dysfunction |
III |
Moderate dysfunction |
IV |
Moderately severe dysfunction (symmetry at rest) |
VSevere dysfunction (asymmetry at rest)
VI |
Total paralysis |

The Facial Nerve and Facial Reanimation |
219 |
|
|
|
|
Table 35.1. The primary muscles of facial expression, their |
|
|||
innervation (branch of facial nerve) and function |
35 |
|||
|
|
|
|
|
Nerve Branch |
Muscle |
Action |
|
|
Temporal |
Anterior auricular |
Pulls ear forward |
|
|
|
Superior auricular |
Pulls ear up |
|
|
|
Corrugator |
Pulls eyebrows inferomedially |
|
|
|
Procerus |
Pulls eyebrows downward |
|
|
|
Occipitofrontalis |
Moves scalp forward |
|
|
Temporal-zygomatic |
Orbicularis oculi |
Closes lids, squinting |
|
|
Zygomatic-buccal |
Zygomaticus major |
Elevates corner of mouth |
|
|
Buccal |
Zygomaticus minor |
Elevates upper lip |
|
|
|
Buccinator |
Pulls mouth laterally (“smile”) |
|
|
|
Levator labii superioris |
Elevates upper lip and |
|
|
|
|
nasolabial fold |
|
|
|
Orbicularis oris |
Closes and compresses lips |
|
|
|
Nasalis |
Flares nostrils |
|
|
|
Levator anguli oris |
Elevates corners of mouth |
|
|
Buccal-marginal |
Depressor anguli oris |
Pulls corners of mouth downward |
|
|
Mandibular |
Depressor labii inferioris |
Pulls lower lip downward |
|
|
Marginal mandibular |
Mentalis |
Pulls chin upward |
|
|
Cervical |
Platysma |
Pulls corners of mouth downward |
|
|
Posterior auricular |
Posterior auricular |
Pulls ear backwards |
|
|
|
Occipitofrontalis |
Moves scalp backwards |
|
|
Long-standing paralysis (greater than 2-3 years) will result in atrophy and fibrosis of the facial muscles and the inability to regain function purely by reinnervation. In these cases a muscle transposition or transplant procedure is required.
The goal of the patient is important to consider. Older patients may be content with achieving static facial symmetry at rest, whereas younger patients usually desire a dynamic repair that will allow them to smile.
Direct Nerve Repair
This is the most effective procedure for reanimating the paralyzed face. It is contingent on the adequate function of the target muscles. One should not attempt to restore function to a muscle that has been paralyzed for over 3 years solely by reinnervating it. In the past, many surgeons advocated waiting at least 3 weeks prior to nerve repair. It is now known that immediate repair of an injured facial nerve yields the best results. In direct nerve repair, an attempt is made to align the fascicles. Once proper orientation of the two stumps is achieved, the perineurium is sewn together followed by the epineurium using 9-0 silk. Smaller nerves in the distal branches can be repaired with a single full-thickness suture. If the stumps of the nerve have a neuroma or appear crushed, the nerve ends should be “freshened” until normal appearing nerve is evident. Direct repair should be undertaken only if a tension free repair is possible. Outcomes are directly correlated to the age of the individual, with younger patients faring far better than older ones.

220 Practical Plastic Surgery
|
Nerve Grafting |
|
|
Autogenous sensory nerve grafts should be used when there is a gap in the facial |
|
35 |
||
nerve that cannot be primarily repaired. The length of the graft should be about |
||
|
||
|
20% longer than the gap. The graft must also be placed in a tissue bed that is free of |
|
|
scar. Ipsilateral cervical plexus nerves are the first choice, followed by the contralat- |
|
|
eral cervical plexus. These usually can provide adequate length (about 10 cm) when |
|
|
several nerves are sewn together. If greater length is needed (e.g., for cross-face graft- |
|
|
ing described below), the sural nerve can provide up to 40 cm of length. A number |
|
|
of “sleeves” have been designed to cover the suture lines. These range from simple |
|
|
silastic tubes to collagen tubes lined by Schwann cells. Most of these have not dem- |
|
|
onstrated any significant benefit. |
|
|
The classic teaching is that peripheral nerve axons regrow at a rate of about 1 mm |
|
|
per day. However, this does not take into account the time required for the reinnervated |
|
|
muscle to regain tone and function. For most patients, return of facial movement |
|
|
takes 1-2 years depending on the length of the graft. Movement usually begins at the |
|
|
oral commissure followed by motion around the eyes and the cheek. The muscles of |
|
|
the forehead and lower lip, however, usually do not regain much movement. |
Nerve Transfer
This technique is employed when direct repair or grafting is not possible. This may be due to the absence of the main trunk of the facial nerve or in cases of intracranial nerve damage. It requires adequate mimetic muscle function and an intact peripheral nerve stump. It involves transferring one of the other cranial motor nerves, most commonly the hypoglossal nerve. Other nerves that have been used include the phrenic, accessory or glossopharyngeal nerve. Nerve transfer will be successful in most patients, even though it often results in mass movement. Following the cross-over procedure, the donor nerve target muscle loses some of its bulk and function. This is usually not a major issue since the tongue receives innervation from several nerves. One disadvantage of this technique is that eating and speaking can produce involuntary motion in the face.
Cross-Facial Nerve Grafting
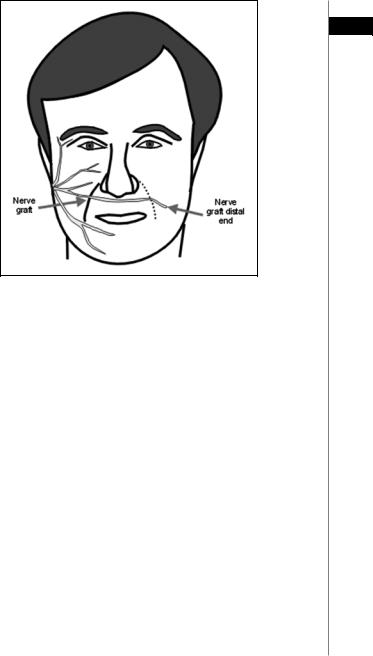
This technique employs a nerve graft (typically the sural nerve) that acts as a conduit for motor axons from the normal, contralateral facial nerve (Fig. 35.2). Usually a single graft is used; however some surgeons will use multiple grafts from the intact contralateral divisions to the corresponding paralyzed ones. Cross-face grafting can be performed in a single stage or as a two-stage procedure. The advantage of the two-stage procedure is that it allows the surgeon to verify that the axons have successfully grown to the opposite side before connecting the nerve to the injured side. In the initial procedure, the desired branch is identified on the normal side and confirmed with a nerve stimulator. The sural nerve graft is sewn to it and then tunneled subcutaneously either above the upper lip or below the lower lip. A clip is attached to the end of the nerve graft. The second procedure is performed about 12 months later, after a Tinel’s sign is present at the distal end of the nerve graft. Any neuroma evident should be resected prior to sewing the nerve graft into the injured nerve stump. The disadvantages of the cross-face graft include an additional donor site in the leg, violating the normal side of the face, two or more suture lines for the axons to cross, a long interval until return of function and reduced motor output from the donor side.
The Facial Nerve and Facial Reanimation |
221 |
35
Figure 35.2. Cross-face nerve graft. Segments of the cervical plexus or the sural nerve are used as a conduit for motor axon regrowth from the normal side. Reprinted with permission from Microsurgeon.org.
Static Suspension Procedures
These procedures involve suspending structures in the face into static symmetry with the contralateral side. They provide no dynamic return of function. Suspension of the eyelids, nares, oral commissure and lower lip has been described. Traditionally, fascia lata slips were used, however recently suture suspension (e.g., contour threads), and slings made of Goretex® and Alloderm® have been described. Suspension procedures can be performed alone or in combination with muscle transfers.
Local Muscle Transposition
This technique is employed when there has been longstanding paralysis and the muscles of facial expression have atrophied and fibrosed. In addition, local muscle transposition should be considered if additional mimetic function at a specific site is required. The masseter and temporalis muscles are the two most commonly used for transfer. Transposition of the platysma and sternoclidomastoid muscles have also been described; however they have poor excursion compared to the muscles of mastication.
The temporalis may be transposed by transecting its origin on the skull along with a rim of epicranium which will serve as an anchor for sutures (Fig. 35.3). The muscle can be split longitudinally creating several slips. These may be transposed to the upper and lower eyelids, the ala, the mesolabial fold, and the upper and lower lips. Overcorrection should be performed by sewing the slips under tension. The depression left from removal of the temporalis can be repaired using a silastic block. The temporalis can also be detached from its insertion into the condyle and sewn into the oral commissure or nasolabial fold.
