
Solid-Phase Synthesis and Combinatorial Technologies
.pdf9.1 PHARMACEUTICAL APPLICATIONS 453
In vivo screening methods to determine a pharmacokinetic profile are more difficult to automate but are more significant in that their result is the synthesis of ADME properties and can safely be used to judge the compounds’ likeliness to become an ADME-friendly drug. Recent reports (64–69) have introduced cassette dosing methods to simultaneously administer mixtures of compounds. To date, a maximum of 10 components have been administered together, and their pK profiles were determined by analyzing the MS spectra of biological samples; a recent paper (70) reported a higher, 64-component-based cassette dosing study. Even though the screening throughput is increased, significant technological improvements are needed to obtain true HTS in vivo ADME methods; a review has recently summarized the state of the art of cassette dosing (71) and should be consulted by the interested reader. Currently, their use in lead optimization is possible, while their use in lead identification would require a much larger throughput.
In vitro studies have mostly been concerned with absorption and metabolism. The use of CACO2 monolayer cells to mimic intestinal absorption of drugs is known and validated (72), and methods to automate the assay have been reported using either CACO2 cells (73, 74) or immobilized artificial membranes (75). Papers dealing with small mixtures of peptoids (76) and with large mixtures of peptides (77) have been presented using LC (76) or MS (77) techniques to detect the permeable compounds. Improvements are to be expected in the selection of new, more user-friendly cell lines, such as MDCK (madin darby canine kidney), in the identification of reliable and sensitive detection methods and in the validation of artificial, stable lipid membrane models to allow an easier automation of the screening. Metabolism-based HTS could be realized using either fresh tissue preparations from human or animal liver or purified enzymes responsible for metabolic effects. With drug metabolism being a multivariate effect, the former format would be more useful but more difficult to set up considering the instability of tissue preparations and their fast and irreversible loss of enzymatic activity (64). Nevertheless, medium-throughput screenings using an LC-MS separation– identification technique for metabolites capable of processing 96 samples in 13 h have been reported (78) together with other relevant examples (79, 80). The use of stable, isolated cytochrome P450 xenobiotic-metabolizing enzymes is much more automation friendly, and the screening results can be used to safely rationalize and predict metabolic effects. Some examples and validation studies have been reported recently (81–83); an intriguing report based on ultrafiltration mass spectrometry claimed a throughput of 20–60 compounds per hour using rat liver microsomes (84).
ADME HTS screens have also been covered in several recent reviews (72, 85–90) that extensively comment on future trends and possibilities to improve the impact and the performance of such tools. The use of such screening as a primary filter, rather than a late optimizing tool, can be envisaged for most of the in vitro or in vivo screening formats. This will contribute to focus significant efforts in drug discovery on valuable candidates, with higher chances to be successfully developed as novel drugs, at an ever earlier stage.
High-throughput toxicological assays to determine the issues of molecules still in a relatively early phase of the drug discovery process are extremely important, and several academic and private groups are active in this field to develop predictive
454 APPLICATIONS OF SYNTHETIC LIBRARIES
models, new high throughput tests and to rationalize the available information in a toxicity database. Several in vitro (91–93) and in vivo (94) toxicological HTSs have been recently reported, and even an in silico approach where the predicted toxicity of druglike substances was used together with HTS has appeared (95, 96). This field was exhaustively covered by several reviews (97–100).
The determination of the physicochemical properties of a molecule, or of an array of molecules, is also extremely important in drug discovery; in fact, the so-called developability of a drug cannot take place if the compound has problems related to its solubility or to its lipophilicity at physiological pH. High-throughput screening assays able to provide an early estimation of these properties for discrete, focused libraries or collections of compounds were reported using several assay formats and detection techniques. Turbidimetry was used to determine solubility (101), while flow injection– capillary electrophoresis (FI-CE) was used to determine the dissolution process of compounds with a throughput of 60 compounds per hour (102); the chromatographic hydrophobicity index of compounds was determined and validated as a higher throughput good alternative (50–100 compounds per day) to log P/log D (103). Penetration through biological barriers, and especially through the blood-brain barrier, has been the subject of both theoretical (104) and experimental research (69). A recent review (105) summarizes most of the current trends in physicochemical HTS and illustrates the use of computational tools to predict and to rationalize the data from HTS.
9.2 AGROCHEMICAL AND FOOD-RELATED APPLICATIONS
9.2.1 General Considerations
The areas where combinatorial technologies impact strongly in the drug discovery process (i.e., the phase of lead identification and optimization) are essentially the same for agricultural and food research. Opportune, relevant targets must be isolated and the necessary adjustments, in terms of desired properties of the active compounds (e.g., physicochemical properties and toxicity), must be made on the basis of the selected agrochemicals or food-related application. It is thus surprising that very few reports in these areas have appeared in the literature. Apart from the example reported in the next section about agrochemicals, Wong et al. (106) isolated peptide ligands for barley α-amylase using phage display libraries; peptide libraries were used to identify anti-phytopathogenic fungi (107) and were designed to identify novel pesticides (108); researchers from FMC Corporation (109) reported the synthesis and the screening of a discrete library of pyrazolopyridines as insecticidals; researchers from Zeneca (110, 111) reviewed the future trends in fungicide/agricultural research, mentioning the synthesis and screening of primary, synthetic organic libraries without divulging any structural information; Jansson et al. (112) reported an evaluation of available HTS assays to screen for novel insecticides; Petsko et al. (113) reviewed the theoretical opportunities provided by combinatorial technologies in the search of novel pesticides. Only two recent papers (114, 115) introduced the use of combinatorial technologies to prepare a flavor library and to characterize its components in terms of their odor

456 APPLICATIONS OF SYNTHETIC LIBRARIES
herbicidal leads on a whole-plant assay. The synthesis of L17 and its structure are reported in Fig. 9.28.
A solid-supported reagent, 9.67, was used to activate the monomer set M1 (60 carboxylic acids, step a, Fig. 9.28) added to the solid support as 6 mixtures of 10 representatives. The activated ester pools 9.68 were reacted with the monomer set M2 (amines and primary alcohols, step b, Fig. 9.28) and the library L17 was recovered, after filtration, as a set of 10-member pools with yields varying from 60 to 90% (mass recovery). Screening and deconvolution produced 9.69, an active compound of interest (Fig. 9.28, bottom). Its structure made it suitable for further optimization via focused library efforts, while its similarity to known bleachers also pointed toward a specific mechanism of action (118). No further efforts were reported by the authors.
9.3 APPLICATIONS TO COMBINATORIAL REACTION OPTIMIZATION
9.3.1 General Considerations
The paradigm of many compounds (a library) prepared simultaneously (combinatorially) and tested rapidly (HTS format) to find a specific activity is the core of combinatorial technologies. This same paradigm can also be applied when a single compound is prepared but a set of reaction variables (a library) are simultaneously
(combinatorially) modified and the reaction outcome is rapidly measured (HTS format) to find the optimal reaction conditions. This multivariate, simultaneous optimization of reaction conditions can be used to accelerate the SP chemistry assessment aimed toward the synthesis of an SP library as recently proved using a limited example (118) (see also Sections 6.1.2 and 6.3.5). A typical example of a biased/focused approach involves tens/hundreds of reaction conditions. Theoretically, though, the exploration of a larger set of new, unprecedented, diverse reaction conditions on a common set of reagents to promote novel reactions could represent the primary/diversity-based approach aimed at more fundamental chemistry research. A primary approach has not been exploited as of today, but it could be instrumental in creating a sort of chemical reaction database allowing the chemist to be more predictive in selecting experimental conditions for a given transformation. Complex encoding strategies could even be used to optimize reaction protocols, as foreseen previously (120).
9.3.2 An Example: Rapid Optimization of the Synthesis of an ICE Inhibitor
Warmus et al. (121) recently reported the multivariate optimization of reaction conditions for the synthesis of a known class of ICE (interleukin-1β converting enzyme) inhibitors, represented by compound 9.70. The structure of the compound and the synthetic route, starting from the key bromide intermediate 9.71, are reported in Fig. 9.29 (the acetylvaline moiety of 9.70 was replaced during the optimization with an Fmoc carbamate).
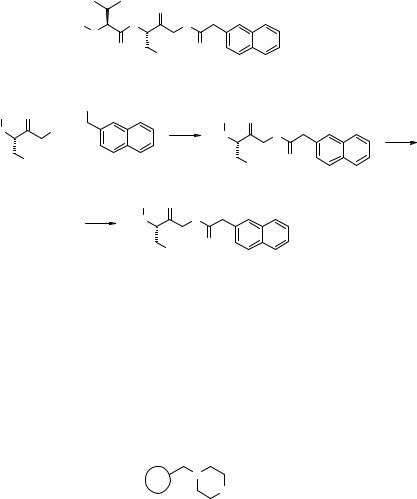
Coupling of 9.71 with carboxylic acid 9.72 (step a, Fig. 9.29) was performed in a set of 82 reaction conditions (parallel reaction library L18, Fig. 9.30). The base and


9.3 APPLICATIONS TO COMBINATORIAL REACTION OPTIMIZATION |
459 |
KF as the best basic reagents and towards DMF as the best solvent. An accurate determination of yields and purity of 9.73 in all reaction vessels selected entry 43 (TEA–DMF) as the best compromise for step a, Fig. 9.29. A similar optimization was performed for the t-butyl ester hydrolysis (step b, Fig. 9.29), creating the 39-member reaction library L19 by permutations of acidic reagents and solvents, and addition of adjuvants (Fig. 9.30). The screening outcome (Table 9.3) highlighted the poor performances of ion-exchange resins (no reaction) and TFA (unclean product) to prepare 9.74, and selected entry 7 (HCl/EtOAc) as the best reaction conditions to obtain clean 9.74 (CSA (camphor sulfonic acid) actually performed slightly better, but the reaction work-up was less automation friendly). The whole manual optimization process required three to four days, and the best reaction conditions were used directly to produce a 590-member discrete library, which met the >75% purity cutoff (78).
Among other similar reports, Bray et al. (122) optimized the SP reductive amination of a model ketone with three primary amines using a 56-member reaction library with simultaneous variation of amines, reducing agent concentrations, pH, and solvents. Gayo and Suto (123) optimized the coupling of an acyl chloride with benzylamine in solution using a 30-member reaction library with simultaneous variation of the
TABLE 9.3 Yields and Purity of 9.74 from Parallel Reaction Library L19: Selected Entries
|
|
|
|
|
Reaction |
Entry |
Acid |
Solvent |
Adjuvant |
Concentration |
Outcomea |
|
|
|
|
|
|
1 |
TFA |
DCM |
b |
20% |
100/88 |
|
|||||
4 |
TFA |
DCM |
PhSMe |
50% |
80/65 |
5 |
TFA |
DCM |
Et3SiH |
50% |
100/65 |
6 |
TFA |
DCM |
m-Cresol |
50% |
100/49 |
7 |
HCl |
AcOEt |
b |
1M |
48/98 |
|
|||||
8 |
HCl |
Toluene |
b |
1 M |
37/97 |
|
|||||
9 |
HCl |
Dioxane |
b |
1 M |
29/97 |
|
|||||
10 |
HCl |
THF |
b |
1 M |
24/98 |
|
|||||
11 |
HCl |
Dioxane |
10% H2O |
1 M |
NR |
12 |
TsOH |
Toluene |
b |
Solid |
70/63 |
|
|||||
14 |
TsOH |
DCM |
PhSMe |
Solid |
65/88 |
16 |
CSA |
THF |
b |
Solid |
74/98 |
|
|||||
18 |
CSA |
THF |
H2O |
Solid |
32/99 |
23 |
IRP-64 resin |
AcOEt |
b |
Solid |
NR |
|
|||||
27 |
IRC-58 resin |
AcOEt |
b |
Solid |
NR |
|
|||||
31 |
DOWEX 50 |
AcOEt |
b |
Solid |
NR |
|
|||||
|
Resin |
|
|
|
|
35 |
Amberlite IR |
AcOE |
b |
Solid |
NR |
|
|||||
|
120 resin |
|
|
|
|
39 |
Nafion resin |
AcOEt |
b |
Solid |
NR |
|
aReaction completion (disappearance of 9.73)/purity; NR = no reaction. bAbsent.

9.4 APPLICATIONS TO CATALYSIS |
461 |
Subsequently, in 1996, Burgess et al. (128) reported a high-throughput catalyst screening for a C–H insertion reaction (see next section), widening the application of combinatorial technologies to the fast optimization of catalytic conditions for a given reaction. Since then, many papers and reports have appeared regarding synthetic organic libraries aimed toward the discovery of new catalyst/ligand systems. These are covered in this section through several examples and are thoroughly referenced. Other approaches regarding inorganic libraries of catalysts, catalytic antibodies, or molecularly imprinted polymers to catalyze organic reactions are among the main topics of the next two chapters.
The most obvious approach utilizes parallel synthesis to prepare arrays containing different catalytic systems, testing them on a single chemical transformation and measuring their efficiency. This is done varying the catalyst, or the ligand, or both of them simultaneously. Three examples of this approach are reported in Sections 9.4.2, 9.4.3 (catalytic system libraries), and 9.4.4 (ligand library). A specular approach employs an array of chemical substrates to determine the specificity and the general efficiency of a given catalytic system and is exemplified in Section 9.4.5. Finally, two intriguing examples of SP pool libraries, screened via either deconvolutive methods (Section 9.4.6) or chemical encoding (Section 9.4.7), determine the activity of a catalytic system using high-throughput mix and split synthetic methodologies. These examples are followed by an exhaustive list of references, which are also summarized in several recent reviews (129–137).
9.4.2 An Example: Screening of a Library of Catalytic Systems for a C–H Insertion Reaction
Burgess et al. (128) reported the catalyst screening of a 96-member array of catalytic systems L20 on a C–H insertion reaction of substrate 9.77 (Fig. 9.32), a transformation usually catalyzed by rhodium (138) or copper salts (139) in the presence of chiral ligands (140). The stereochemical outcome was measured on the diastereomeric couple 9.79–9.80, obtained following uncatalyzed oxidation of 9.78 (Fig. 9.32), to simplify the determination of the chiral products while evaluating the stereoselectivity of the tricycle formation. The stereoselectivity of the C–H insertion was not significantly influenced by the presence of the (L)-methyl ester (128).
The composition of L20 is reported in Fig. 9.33. Five chiral ligands were used, including three chiral bis(oxazolidines) 9.81 (141), 9.82 (142), and 9.83 (143); sparteine 9.84 and a salen-type compound 9.85 (144). Seven metal ions were used with the different ligands, silver, and scandium as well as the assessed copper and rhodium salts were trialed with each ligand, while gold, ytterbium, and lanthanium salts were employed more conservatively with only one or two ligands each. The 24 combinations of catalyst and ligand were tested in four solvents. Each catalytic system was tested on a 10-mg sample of 9.77 using small quantities of ligands and catalysts (10% in moles, 0.6–1.5 mg) in a 200- L solution stirred at rt or at 10 °C. The reaction outcome was measured by HPLC, the diastereomeric couple 9.79–9.80 being sufficiently distinguishable. The whole process of catalytic reactions, work-up, and purification of samples and analytical determination of reaction products was completed in a few days for the 96-member array L20.

462 APPLICATIONS OF SYNTHETIC LIBRARIES
O |
O |
|
O |
O |
|
|
|
|
|||
|
|
catalyst |
|
H |
|
|
|
|
|
|
|
|
N2 |
|
N |
O |
|
|
|
|
|
|
|
N |
O |
|
9.78 |
O |
|
|
|
|
|
||
9.77 |
O |
|
|
|
|
|
uncatalyzed |
O |
|
O |
|
|
|
O |
O |
||
|
DDQ oxidation |
|
+ |
||
|
|
|
|
||
|
|
|
|
|
|
|
|
|
O |
|
O |
|
|
N |
|
N |
|
|
|
9.79 |
O |
9.80 |
O |
|
|
|
|
Figure 9.32 Catalyzed C–H insertion on azo compound 9.77 and uncatalyzed oxidation of 9.78 to tricyclic structures 9.79 and 9.80.
The observed stereochemical outcome of the C–H insertion varied from 1 : 2.4 (9.80 favored) to 5.9 : 1 (9.79 favored), with many examples of scarce asymmetric induction. The use of THF generally led to higher stereoselection than the other solvents. The most significant results of the screening experiments are reported in Table 9.4. Seventeen catalytic combinations gave encouraging results and were repeated on a larger scale to reconfirm the observed activities. Several data were not reconfirmed, probably because of experimental errors and heterogeneities in the small-scale primary screening. The already known catalytic system (entry 18) was surpassed in terms of diastereomeric ratio (entry 19) and of both diastereomeric ratio and reaction yield (entry 20). The use of silver ion as C–H insertion catalyst was novel and highlighted the potential of combinatorial catalyst screening in affording relevant, unpredictable new catalytic systems.
An increased reliability of the results generated in the primary screening, an increased throughput, and the use of larger sets and libraries of catalytic systems were the main issues emerging from this pivotal contribution. The following examples will include more recent reports that partially address these concerns.
9.4.3 An Example: Screening of a Library of Catalytic Systems for a Hydrosilylation Reaction
Cooper (145) recently reported the use of dye-containing substrates as screening tools for the rapid evaluation of different catalytic systems. Hydrosilylation was chosen as
