

IV.4 Allylpalladation and Related
Reactions of Alkenes, Alkynes, Dienes,
and Other -Compounds
TAKASHI TAKAHASHI and TAKAYUKI DOI
A. INTRODUCTION
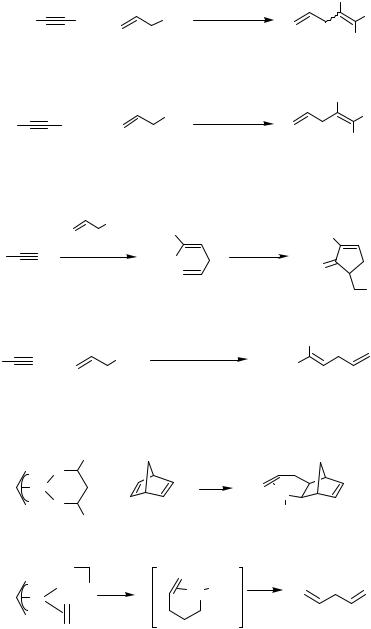
Allylic halides react intermolecularly with alkynes in the presence of palladium(II) catalysts (Scheme 1).[1],[2] The products are vinylic halides, which have further been elaborated by Ni(CO)4-mediated carbonylation[3] and Pd-catalyzed cross-coupling reaction, for example, with organotin compounds (Scheme 2).[4]
There are only a few cases of an intermolecular reaction of a -allylpalladium complex with an alkene. The carbopalladation of norbornadiene with a stoichiometric amount of a -allylpalladium complex to yield an allyl-substituted -norbornenylpalladium species has been reported.[5] In the context of mechanistic studies of Pd-catalyzed alkene dimerization, the insertion of ethylene into a cationic -allylpalladium complex was also reported as a stoichiometric reaction in 1996 (Scheme 3).[6]
B. INTRAMOLECULAR REACTION OF A -ALLYLPALLADIUM
COMPLEX WITH ALKENES AND DIENES
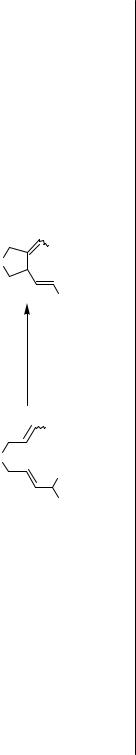
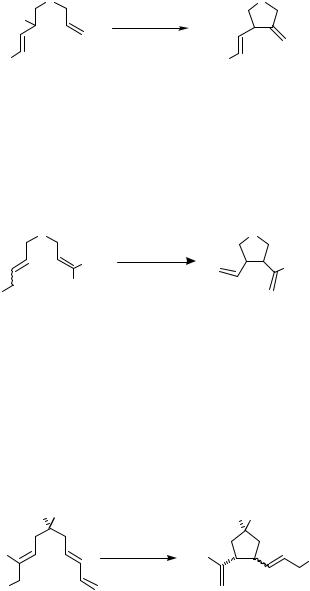
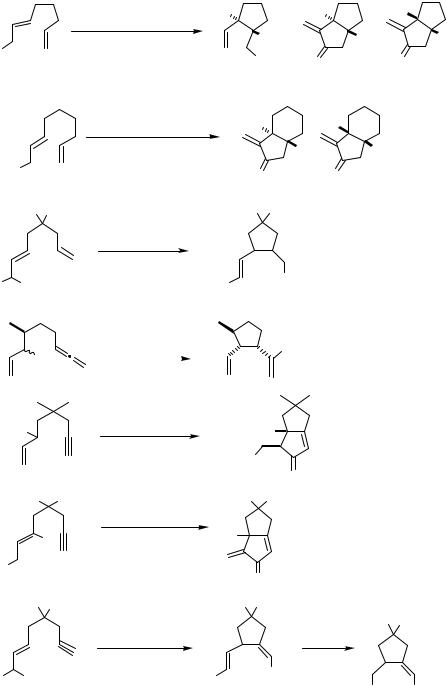
In 1987, Oppolzer and Gaudin reported an intramolecular version of the reaction of a - allylpalladium complex with an alkene.[7] The reactions of various 2,6-octadienyl acetate derivatives were achieved in the presence of Pd(dba)2-PPh3 at 70–80 °C leading to 2-vinyl- 1-alkenylidenecyclopentane derivatives in yields ranging from 20% to 98% (Schemes 4 and 5). With one or two methyl groups attached at the alkenyl terminus, the acyclic alkenylallyl acetates under Pd(II) catalysis cyclized to yield 1-alkenyl-2-vinylcyclopentane derivatives (Scheme 6).[7]–[11] A -allylpalladium intermediate generated from an allyl ester can also intramolecularly carbopalladate a terminal diene or allene unit (Scheme 7, for the latter see also Sect. IV.7).[12],[13] These cyclizations lead to new -allylpalladium intermediates, which are eventually trapped by acetate to yield allyl acetates.
Handbook of Organopalladium Chemistry for Organic Synthesis, Edited by Ei-ichi Negishi ISBN 0-471-31506-0 © 2002 John Wiley & Sons, Inc.
1449
1450 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
||||||
|
|
|
|
|
|
|
CH3 |
|
Ph |
|
Me + |
Cl |
PdCl2 (PhCN)2 |
|
Ph |
|
|
20 °C |
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|
Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
80% |
|
|
|
|
|
|
|
SiMe3 |
|
n-C6 H13 |
|
+ |
Cl |
PdCl2(PhCN)2 |
|
n-C6H13 |
|
|
|
|
|
|
||
|
|
SiMe3 |
|
20 °C |
|
Cl |
|
|
|
|
|
|
|
||
|
|
|
|
|
91% |
|
|
|
|
|
|
|
>98% stereoselective |
||
|
|
|
|
|
|
||
|
|
|
|
Scheme 1 |
|
|
|
|
|
|
Br |
Me3Si |
|
|
|
|
|
|
PdCl2(PhCN)2 |
Ni(CO)4 |
|
Me3Si |
|
|
|
|
|
MeOH, Et N |
|
||
|
Me3Si |
|
|
Br |
|
3 |
|
|
|
25 °C 62% |
MeCN, 30 °C |
O |
|||
|
|
|
|
||||
|
|
|
|
|
65% |
CO2 Me |
|
|
|
|
|
|
|
||
|
|
|
|
5 mol % PdCl2(MeCN)2, |
|
Ph |
|
|
n-Bu |
+ |
Br |
20 |
°C, 2 h |
|
|
|
|
|
n-Bu |
|
|||
|
|
then, PhSnBu3, HMPA, |
|
||||
|
|
|
|
|
|||
|
|
|
|
|
|
||
|
|
|
|
TFP, Bu4 NCl, 80 °C |
|
24% |
|
|
TFP = tris-(2-furyl)phosphine |
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
Scheme 2 |
|
|
|
|
|
|
CF3 |
|
|
|
|
|
|
O |
|
|
|
|
|
|
Pd |
|
+ |
|
|
Pd |
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
L |
|
|
|
|
|
CF3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
+ |
|
+ |
|
|
|
|
PCy3 |
|
PCy3 |
|
|
|
|
Pd |
|
|
|
Pd |
|
|
|
|
> −20 °C |
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 3
The key reactive intermediate in all these cyclizations is an alkene-coordinated cationic -allylpalladium complex (Scheme 8), the formation of which is probably promoted by protonation of the acetoxy group when allylic acetates are used as staring materials.[6],[14] It is thus understandable why acetic acid is mostly used as a crucial solvent. The -alkylpalladium intermediate generated through alkene insertion can undergo
Reference |
[7] |
Ratio |
|
|
|
Yield(%) |
82 |
Y |
R |
Temperature [°C] |
|
X |
70 |
||
|
|
Solvent |
THF |
|
R |
|
|
Y |
X |
Catalyst |
A |
|
AcO |
|
|
|
|
R |
H |
|
|
|
2 |
|
|
Y |
-p-MePh) |
|
|
|
2 |
|
|
|
C(SO |
|
|
X |
H |
[7] |
[7] |
[7] |
[7] |
[8] |
[8] |
[8] |
[9] |
[9] |
[9] |
[8] |
[10] |
[10] |
[10] |
|
|
|
|
|
|
|
only (Z/E) |
only |
|
|
|
|
|
|
|
|
|
|
|
|
(Z) |
96:4 |
(E) |
|
|
|
|
20 |
65 |
77 |
75 |
72 |
69 |
72 |
Notnoted |
Notnoted |
57 |
77 |
80 |
98 |
99 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
THF |
MeOH |
AcOH |
AcOH |
AcOH |
AcOH |
AcOH |
AcOH |
AcOH |
AcOH |
AcOH |
AcOH |
AcOH |
AcOH |
A A A A B B B A A C B B C C |
|||||||||||||
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
H H H H H H H |
(Z)-Ph |
(E)-Ph |
(E)-SiMe |
H |
H H H |
||||||||
2 |
|
|
2 |
|
2 |
|
|
|
|
|
2 |
|
2 |
|
|
CMe |
|
|
|
|
|
|
|
||||
Me) |
|
|
|
|
Ph |
|
|
|
|
2 |
Et) |
|
Ph) |
|
|
2 |
Ph |
CH |
Ts-N |
|
|
|
|
||||
C(CO |
|
|
C(COO) |
NCH |
NCO |
|
|
|
N(Ts)CH- |
C(CO |
|
C(SO |
|
2 |
|
|
|
2 |
2 |
|
|
|
|
|
2 |
|
2 |
H |
H |
H H H H H H H |
H |
H |
OAc |
OAc |
OAc |
||||||
Scheme 4
3. toly)-o
P(, 2
Pd(dba)C:
;
4 ) 3 Pd(PPhB:
;
3 PPh3
,
2 Pd(dba)A:
1451
1452 IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION
|
|
Y |
|
|
|
|
Y |
|
|
|
|
XO |
|
Pd(PPh3)4 |
|
|
|
|
|
|
|
|
|
AcOH, 80 °C |
|
|
|
||
|
|
R |
|
|
|
|
R |
|
|
|
|
R |
X |
|
Y |
|
Yield (%) |
Reference |
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
Bz |
|
NCOPh |
81 |
[8] |
|
|
|
|
H |
H |
NSO2-p-MePh |
78 |
[8] |
|
||
|
|
C6H5 |
Ac |
|
CH2 |
49 |
[11] |
|
|
|
|
4-Me-C6H4 |
Ac |
|
CH2 |
67 |
[11] |
|
|
|
|
3,5-(MeO)2-C6H3 |
Ac |
|
CH2 |
94 |
[11] |
|
|
|
|
|
|
Scheme 5 |
|
|
|
||
|
|
Y |
|
|
|
|
Y |
|
|
|
|
|
R1 |
|
AcOH, 80 °C |
|
R3 |
|
|
|
|
|
|
|
|
|
|
||
|
|
AcO |
R2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Substrate |
Y |
R1 |
R2 |
R3 |
Catalyst |
Yield (%) |
trans/cis Reference |
||
(E) |
NCOCF3 |
Me |
H |
H |
B |
76 |
88:12 |
[8] |
|
(Z ) |
NCOCF3 |
Me |
H |
H |
B |
59 |
91:9 |
[8] |
|
(E) |
NCOCF3 |
H |
Me |
H |
B |
67 |
28:72 |
[8] |
|
(Z ) |
NCOCF3 |
H |
Me |
H |
B |
51 |
30:70 |
[8] |
|
(Z ) |
C(SO2-p-MePh)2 |
H |
Me |
H |
A |
91 |
Single isomer |
[7] |
|
(Z ) |
C(SO2-p-MePh)2 |
Me |
Me |
Me |
A |
71 |
Single isomer |
[7] |
|
A: Pd(dba)2, 3 PPh3; B: Pd(PPh3)4. |
|
|
|
|
|
||||
|
|
|
|
Scheme 6 |
|
|
|
||
|
|
R2 R1 |
|
|
|
R2 R1 |
|
|
R3 |
|
Pd(dba)2·CHCl3 |
R3 |
|
OAc |
|
|
|
|
90 °C |
|
|
|
|
|
AcO |
|
[12] |
|
|
|
|
|
|
|
|
|
|
|
|
R1 |
R2 |
R3 |
Solvent |
|
Additive |
Yield (%) |
trans /cis |
SO2Ph |
SO2Ph |
H |
MeCN |
|
None |
60−70 |
2.3:1 |
SO2Ph |
SO2Ph |
H |
MeCN |
AcOH, LiOAc |
87 |
Not noted |
|
SO2Ph |
SO2Ph |
H |
AcOH |
AcOH, LiOAc |
71 |
2.5:1 |
|
SO2Ph |
SO2Ph |
Me |
MeCN |
AcOH, LiOAc |
63 |
1.4:1 |
|
H |
SO2Ph |
H |
MeCN |
AcOH, LiOAc |
68 |
cis only |
|
H |
SO2Ph |
Me |
MeCN |
AcOH, LiOAc |
62 |
cis only |
|
CO2Et |
CO2Et |
H |
AcOH |
|
PPh3 |
75 |
Not noted |
Scheme 7
IV.4 ALLYPALLADATION AND RELATED REACTIONS |
1453 |
|
• |
Pd(PPh3)4 |
OAc |
|
|
||
|
|
AcOH, 80 °C |
|
AcO |
|
64% |
|
|
|
[13] |
|
|
|
Scheme 7 (Continued ) |
|
OAc |
|
|
|
+RO_ |
Pd0 |
|
PdII |
|
PdII |
|
|
|
||
β-hydride |
|
|
|
|
elimination |
|
|
|
|
_Pd—H |
|
|
|
|
|
|
PdII |
|
|
intramolecular alkene |
carbonylation |
transmetallation |
||
insertion |
|
CO |
|
R-SnR3 |
|
|
|
|
Pd |
|
|
|
|
R |
PdII |
O |
Pd |
II |
|
|
|
|
||
Scheme 8
-hydride elimination to terminate the reaction sequence leading to a diene. Domino-type reaction sequences are possible in that another intramolecular alkene insertion, a carbonylation, or a transmetallation forming a further new carbon–carbon bond can occur. These cases are discussed below.
C. STEREOCHEMICAL ASPECTS
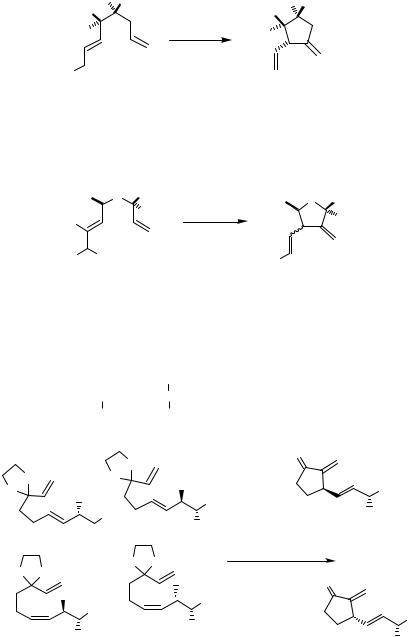
The stereochemical outcome of such intramolecular carbopalladations, which is influenced by the number and nature of the substituents at the various positions of the acyclic system, was examined by several groups (Scheme 9).[8],[15]–[18]
Rather efficient chirality transfer was observed for the alkene insertion into the - allylpalladium complex prepared from various acyclic allylic acetates in the synthesis of the 11-deoxy analog of Stork’s intermediate for the synthesis of prostaglandins (Scheme 10).[19]
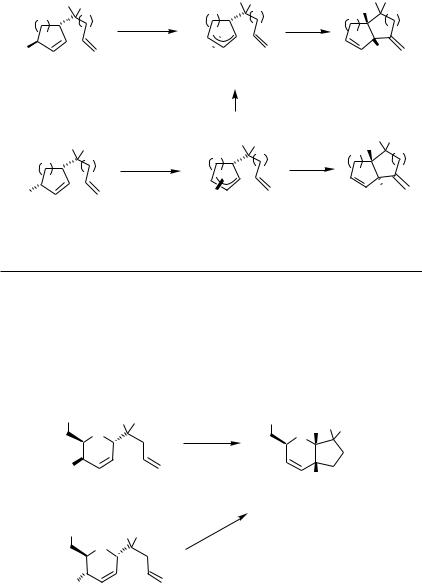
This method was utilized in the preparation of bicyclic compounds consisting of five-, six-, and seven-membered rings. Oxidative addition of allylic acetates to palladium(0) takes place with inversion of configuration and intramolecular alkene insertion into the
1454 |
|
IV |
Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
|
|
|
||||||||||||
|
|
|
|
|
|
|
R3 R4 |
|
|
R 2 R |
3 |
4 |
|
|
|
|
||
|
|
|
|
|
R2 |
|
|
|
|
|
|
|
R |
|
|
|
||
|
|
|
|
|
R1 |
|
|
|
Pd(PPh3)4 |
R 1 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
MeCN, 80 °C |
|
|
|
|
|
|
|||
|
|
|
|
AcO |
|
|
|
|
[15] |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
R1 |
R2 |
|
|
R3 |
R4 |
Yield (%) |
|
(Z)/(E) |
|
|
|
|||
|
|
|
|
OH |
Me |
|
|
H |
H |
75 |
|
4:1 |
|
|
|
|
||
|
|
|
|
OH |
Me |
|
|
Me |
Me |
82 |
|
5:1 |
|
|
|
|
||
|
|
|
|
OH |
Ph |
|
|
allyl |
H |
80 |
|
1.4:1 |
|
|
|
|
||
|
|
|
|
OH |
—(CH2)4 |
— |
H |
75 |
|
1.3:1 |
|
|
|
|
||||
|
|
|
|
|
R4 |
|
Y |
R2 |
|
|
R4 |
|
Y R2 |
|
|
|
||
|
|
|
|
|
|
|
|
R3 |
Pd(PPh ) |
|
|
|
|
|
|
|||
|
|
|
|
1 |
|
|
|
|
|
|
|
3 4 |
|
|
R3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
R |
|
|
|
|
AcOH, 80 °C |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
AcO |
X |
|
|
|
|
X |
|
Yield |
cis/ |
Ref- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
R1 |
|
|
R2 |
R3 |
|
|
R4 |
|
|
|
|
|||||||
|
|
|
|
|
X |
Y |
|
(%) |
trans |
erence |
||||||||
H |
|
C6H12 |
H |
|
|
H |
|
H |
O |
|
75 |
50:50 |
[8] |
|||||
Me SO2-p-MePh |
Me |
|
|
H |
|
H |
CH2 |
|
38 |
1:99 |
[16] |
|||||||
H SO2-p-MePh |
H |
|
|
H |
|
H |
C(CO2Me)2—CH2— 12 |
82:18 |
[17] |
|||||||||
Me SO2-p-MePh |
H |
|
|
H |
|
H |
C(CO2Me)2—CH2— 25 |
98:2 |
[17] |
|||||||||
H |
|
|
|
H |
H |
Allyl— O—CH2 |
|
OAc |
O |
|
88 |
trans only |
[18] |
|||||
H |
|
|
|
H |
H |
|
CH2OCMe2OCH— OAc |
O |
|
84 |
trans only |
[18] |
||||||
H |
|
|
|
H |
H |
|
|
|
|
|
OAc |
CH2 |
|
80 |
trans only |
[18] |
||
|
|
|
CH2OCMe2 OCH— |
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
Scheme 9 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
O |
|
|
O |
|
|
|
||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
||
|
|
O |
|
|
|
OAc |
|
|
|
|
Am |
|
|
|||||
|
O |
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
OAc |
14(R) |
|
Am |
|
12(R) |
|
|
|||||||||
|
|
|
|
14(S) |
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
Am |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
|
|
||
|
|
|
|
|
|
|
|
|
OBn |
1. Pd(PPh3)4 ( 20 mol %) |
|
|
||||||
|
|
|
|
|
OBn |
B |
|
|
|
|||||||||
|
|
|
|
A |
|
|
|
|
AcOH, PhH (2:1) |
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
80 °C, 3.5 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O O |
|
|
|
|
|
|
|
|
||
|
O |
O |
|
|
|
|
|
2. PPTs, acetone |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
OAc |
|
|
|
OAc |
|
20 °C, 2 h |
|
O |
|
|
|
|||
|
|
|
|
|
|
14(S) |
|
Am |
|
|
|
|
|
|
|
|||
|
|
|
14(R) |
Am |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
Am |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
OBn |
|
|
|
|
||||
|
|
|
|
|
OBn |
|
|
D |
|
|
|
12(S) |
|
|
||||
|
|
|
|
C |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
Configuration of |
|
|
|
OH |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Starting Material |
Yield (%) |
|
Major Product |
|
Chirality Transfer (%) |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
A |
|
|
|
65 |
|
|
|
12(R) |
|
95 |
|
|
||
|
|
|
|
B |
|
|
|
50 |
|
|
|
12(S) |
|
95 |
|
|
||
|
|
|
|
C |
|
|
|
64 |
|
|
|
12(R) |
|
81 |
|
|
||
|
|
|
|
D |
|
|
|
56 |
|
|
|
12(S) |
|
84 |
|
|
||
Scheme 10
IV.4 ALLYPALLADATION AND RELATED REACTIONS |
1455 |
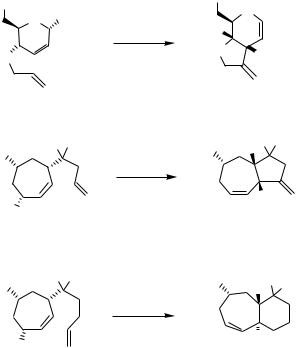
resultant -allylpalladium complex proceeds with retention of configuration to provide bicyclic products in a stereospecific manner (Scheme 11).[20]–[23] However, when the-allylpalladium moiety produced on a ring is trans-positioned with respect to the alkene tether and thus can only insert into the double bond via a highly strained transition structure, epimerization may occur before the bicyclic compound is formed (entry 5 in
Scheme 11).
|
E |
E |
|
|
|
E |
E |
E |
E |
|
|
|
|
|
|
H |
|
||
|
|
|
|
|
|
|
|
|
|
|
m |
|
n |
|
|
m |
n |
m |
n |
|
|
7 mol % |
|
|
|
|
|||
AcO |
|
|
|
|
|
|
|
||
|
|
Pd(PPh3)4 |
|
Pd |
|
H |
|||
|
trans |
|
AcOH, 70 °C |
|
|
|
cis |
|
|
|
|
|
[20] |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
m = 2 |
epimerization |
|
|
|
|
|
|
|
|
n = 1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
E |
E |
E |
E |
|
E |
E |
|
|
|
H |
|
||
|
|
|
|
|
|
|
|||
|
m |
|
n |
|
|
m |
n |
m |
n |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
AcO |
|
|
|
|
|
Pd |
|
|
H |
|
|
|
cis |
|
|
|
|
trans |
|
|
|
|
|
|
|
|
|
|
|
Entry |
Starting Material |
m |
n |
Yield (%) |
Ratio (cis/trans) |
||||
1 |
|
|
trans |
|
1 |
1 |
67 |
>99:1 |
|
2 |
|
|
trans |
|
2 |
1 |
84 |
>98:2 |
|
3 |
|
|
trans |
|
2 |
2 |
60 |
>99:1 |
|
4 |
|
|
trans |
|
3 |
1 |
73 |
99:1 |
|
5 |
|
|
cis |
|
2 |
1 |
55 |
>98:2 |
|
6 |
|
|
cis |
|
2 |
2 |
69 |
5:95 |
|
7 |
|
|
cis |
|
3 |
1 |
80 |
2:98 |
|
|
OAc |
E |
E |
|
|
OAc |
E E |
|
|
|
|
|
O |
|
|
|
H |
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
Pd(PPh3)4 |
|
|
|
|
|
AcO |
|
|
|
AcOH, 70 °C |
|
|
||
|
|
|
|
|
83–89% |
H |
E = CO2Et |
||
|
|
|
|
|
[21] |
|
|
|
|
|
OAc |
E |
E |
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
AcO
Scheme 11 (Continued)
1456 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
OAc
O OiPr
TsN
E E
TBDPSO
AcO
E E
TBDPSO
AcO
|
OAc |
|
Pd(PPh3)4 |
|
O |
|
|
|
AcOH, 80 °C |
H |
|
82% |
TsN |
H |
[22] |
|
|
|
|
TBDPSO H E E
Pd(PPh3)4
AcOH, 70 °C
87% H E = CO2Et
[23]
TBDPSO |
|
E |
E |
|
|
H |
|
||
Pd(PPh3)4 |
|
|
|
|
AcOH, 70 °C |
|
|
|
|
54% |
H |
|
E = CO2Me |
|
[23] |
|
|
|
|
|
|
|
|
|
trans/cis = 93:7 |
|
|
|
|
Scheme 11 |
|
|
|
|
D. DOMINO AND CASCADE REACTIONS INITIATED BY-ALLYLPALLADIUM INSERTION INTO ALKENES OR ALKYNES
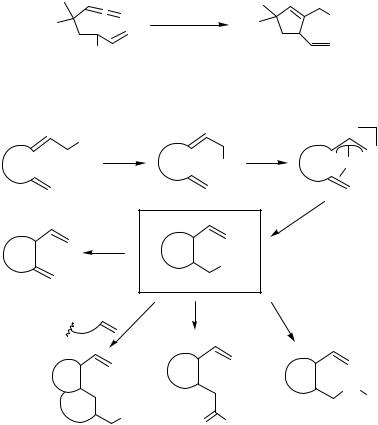
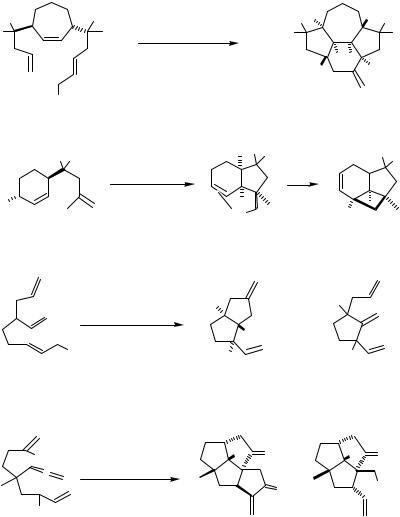
The Pd-catalyzed cyclization of alkenyland alkynyl-tethered allylic acetates gives - alkyland alkenylpalladium intermediates, respectively, which can undergo further intramolecular alkene insertion, carbonylation, and transmetallation, respectively, to form additional carbon–carbon bonds (Scheme 8), as exemplified for such cascade reactions with insertion of a tethered alkene in Scheme 12.[13],[24]–[27]
Alkene and alkyne insertions followed by carbonylation are shown in Schemes 13,[10],[28]–[33] 14,[34] and 15.[21],[35],[36] Interestingly, alkene insertion occurs faster than CO insertion. In several cases, the carbonylation was succeeded by hydrolysis to provide the corresponding acid or yet another carbon–carbon bond formation in the same operation.
Examples of alkene and alkyne insertions followed by transmetallation with organotin compounds as well as boranates, and eventually reductive elimination are shown in Scheme 16.[10],[18],[37]–[39] These reactions do not only require that the transmetallation process as outlined in Scheme 8 be faster than -hydride elimination, but also that the alkene insertion prevails over allylic coupling.
IV.4 ALLYPALLADATION AND RELATED REACTIONS |
1457 |
E |
|
E |
Pd(dba)2, TFP |
E H |
H E |
E |
|
E |
AcOH, 110 °C |
E |
E |
|
|
|
50% |
|
H H |
|
|
|
[24] |
|
|
|
|
|
H |
H |
|
OAc |
|
TFP = tri-(2-furyl)phosphine |
E = CO2Me |
||
|
|
|
|||
E |
E |
Pd(OAc)2, PPh3 |
H E E |
H E E |
|
|
|
PhOMe, NaO2C-H |
|
|
|
AcO |
|
110 °C, 62% |
H |
|
|
|
|
[25] |
H H |
||
|
|
|
Pd |
||
L
E = CO2Me
Pd (PPh3)4, AcOH, 80 °C |
H |
|
H |
|
|
+ |
|
||
|
67% |
|
|
|
|
|
H |
|
|
OAc |
[26] |
H |
|
H |
|
|
|||
|
|
|
||
|
|
5 |
: |
1 |
R |
R O |
R |
O |
Pd(PPh3)4, AcOH, CO |
+ |
|
|
• |
|
||
|
75 or 90 °C |
O |
CO2H |
AcO |
[13],[27] |
|
|
|
R = H (22%) |
R = Me (47%) |
|
|
R = Me (30%) |
||
Scheme 12
E. ASYMMETRIC VERSION OF THE ALKENE INSERTION INTO A -ALLYLPALLADIUM COMPLEX
A few examples of asymmetric cyclizations induced by palladium(0) catalysts with various chiral phosphine ligands have been reported, but the achieved enantioselection has been rather low or at best moderate (Scheme 17).[40] Further studies are definitely needed to improve the catalytic asymmetric version of this intramolecular carbopalladation.
1458 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
AcO
AcO
H
Pd(PPh3)4,AcOH, CO (1 atm)
80 °C
80%
[28],[29]
Pd(PPh3)4, AcOH, CO (1 atm)
|
80 °C |
|
50% |
|
[28],[29] |
E E |
Pd2(dba)3 ·CHCl3 |
|
tri-o-tolylphosphine |
|
AcOH, CO, |
|
80 °C, 0.1 h |
|
H |
|
|
H |
H |
|
|
H |
H |
CO2 H |
O |
|
|
O |
62 |
: |
12 |
: |
26 |
H |
|
H |
|
|
|
H |
|
H |
|
O |
|
O |
|
|
1 |
: |
|
3 |
|
E E |
|
|
|
|
|
|
|
|
|
81% |
|
|
|
CO |
H E = CO2Et |
|
AcO |
OAc |
|
|
|
|
|
AcO |
|
|||
|
|
|
[10] |
|
2 |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pd2(dba)3 ·CHCl3 |
|
|
|
|
|
||
|
|
|
|
TFP, AcOH, CO |
|
|
|
|
|
||
|
OAc |
|
|
45 °C |
|
|
CO2H |
|
|||
|
|
|
58% |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[30] |
|
|
|
|
|
|
|
|
|
|
Pd(dba)2, PPh3 |
|
|
|
|
|
||
MeO2CO |
|
|
AcOH, CO (1 atm) |
|
|
H |
|
|
|||
|
|
|
|
|
45 °C |
|
|
|
|
|
|
|
|
|
|
|
56% |
|
|
HO2C |
|
|
|
|
|
|
[31],[32] |
|
|
|
|
|
|||
|
|
|
|
|
|
O |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
E |
E |
|
|
|
|
|
E |
E |
|
|
|
|
|
Pd2(dba)3· CHCl3, PPh3 |
|
|
|
|
|
|||
|
R |
LiCl, CO, THF, H2O |
|
R |
|
|
|
||||
|
70 °C |
R = H (43%) |
|
|
|
|
|
||||
AcO |
|
|
[33] |
R = Me (57%) |
|
|
|
|
|||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
E = CO2M e |
|
||
|
E |
E |
Pd2(dba)3 ·CHCl3 |
|
E E |
|
|
|
|||
|
|
|
|
|
|
E |
E |
||||
|
|
|
tri-o-tolylphosphine |
|
|
|
|||||
|
|
|
|
|
|
Et3 N |
|
||||
|
|
|
AcOH, CO, |
|
|
|
|
||||
|
|
|
|
80 °C, 0.1 h |
|
|
|
MeOH |
|
||
AcO |
OAc |
|
|
|
75% |
AcO |
|
CO2Me |
|
||
|
[10] |
|
CO2Me |
||||||||
|
E = CO2Et |
|
|
|
|
CHO |
|||||
|
|
|
|
|
|
|
|
|
|
||
Scheme 13
