
|
IV.3.1 CASCADE CARBOPALLADATION: TERMINATION WITH ALKENES |
1379 |
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
I |
|
|
|
|
Pd(OAc)2, PPh3 |
BnN |
|
||||||||||||
|
|
|
|
|
Bn |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
N |
+ |
|
|
|
|
C6H6, 80 °C |
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
30% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 20 |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
HO |
|
|
|
Pd(PPh3)2Cl2, |
E |
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Et3N, CuI, MeCN |
|
|
|
OH |
|
||||
E |
|
|
+ |
|
|
|
|
|
|
|
|
reflux, 10−14 h |
E |
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
97% |
|
|
|
|
|
|
|
|
|||||||||||
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[25] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
n-Pr |
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
PdCl2(PPh) |
|
|
|
|
n-Pr |
|
|||||||||
|
|
|
|
|
|
|
Ph |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Et3N, DMF, |
|
|
|
|
|
||||||||
E |
|
|
|
|
|
E |
|
|
|||||||||||||||||||
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
125 °C, 3 h |
|
|
|
|
|
|
|||||||
E |
|
|
|
|
|
|
Pr |
|
|
|
83% |
|
|
E |
|
|
n-Pr |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
n- |
|
|
|
[9] |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
Et |
|
|
|
Pd(PPh3)4 |
|
|
|
|
SiMe3 |
|
|||||
|
|
|
|
|
|
|
SiMe3 |
|
|
|
|
|
Et3N, DMF |
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
E |
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
+ |
|
|
|
|
|
|
|
|
65 °C, 12 h |
|
|
|
|
|
|
||||||||||
|
|
|
Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Et |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
40% |
|
|
E |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
Et |
|
|
|
[9] |
|
|
E |
|
|
|
|||||
Et
E = CO2Me
Scheme 21
D. ALL-INTRAMOLECULAR CASCADE CARBOPALLADATIONS
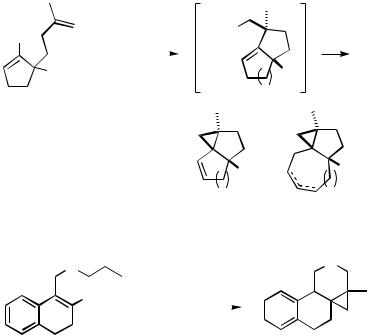
Systems that contain a haloalkene starter, at least one relay, and an alkene or arene terminator in one and the same molecule can undergo a Pd-catalyzed cascade reaction involving two or more intramolecular carbopalladations and termination by dehydropalladation. The increase in molecular complexity is particularly impressive in such cases, in which up to seven new carbon –carbon bonds and also new cycles have been formed in a single procedural step. Various combinations of reactive units and geometries have been applied, and although most of the mechanics are now well understood, a certain flair of unpredictability still remains, especially as one proceeds to unprecedented combinations of ring sizes to be stitched or zipped together. A variety of fascinating cascade reactions consisting of at least one and often more than one intraor intermolecular cross-coupling reaction as well as possibly one or more other reaction types have been developed by several groups. Among the ones that achieve the
1380 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
most striking increase in molecular complexity from starting material to product are the molecular zipper reactions by Negishi,[26] Trost and Shi,[27],[28] and Overman et al.,[29] the triand tetracyclizations of 2-bromodienynes by de Meijere and co-workers,[30] and the carbonylative intramolecular cross-coupling cascades by Negishi and co-workers[31] in which up to seven new carbon –carbon bonds are formed in a single operation.
D.i. Termination by Alkenes
Cascade carbopalladation sequences after attack on an alkene are most commonly terminated by dehydropalladation, if a -hydride is present in a syn-orientation (see above).
For cascades with intramolecular carbopalladations, smaller ring sizes are preferred by entropic terms, 1,1-disubstituted alkenes suitably located in the chain might serve as the relay without the formation of the larger ring size caused by insertion of the terminator.
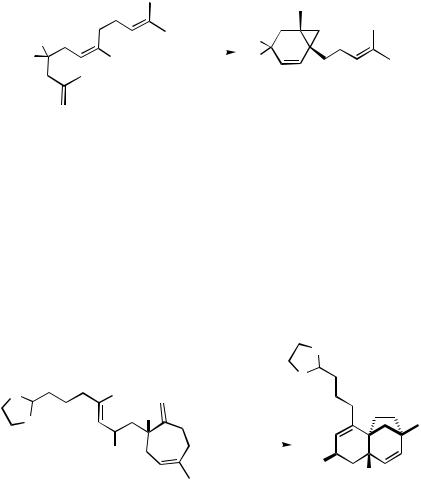
D.i.a. Formation of Cyclopropane Derivatives by Two Successive Intramolecular Carbopalladations. Intramolecular carbopalladation starting from 1,(n 1)-dienes with a suitable leaving group at the 2-position and a substituent at the (n 1)-position of the alkene terminator leads to a neopentylpalladium intermediate, which can only continue the cascade by a 3-exo-trig-carbopalladation to eventually form bicyclo[(n 2). 1.0]alkenes. This sequence works equally well for ring sizes five, six, and seven in the first formed ring (Scheme 22)[32] and even heterocyclic systems can be constructed by this mode (Scheme 22).[33]
|
|
Pd(OAc)2, PPh3 |
|
|
OTf |
|
Na2CO3, Et4NCl, |
TfOPd |
|
|
MeCN, 80 °C |
|
L2 |
|
|
|
|||
|
|
70–73% |
n-4E |
|
( )n-4 |
E |
|||
|
E = CO2Me |
|
||
|
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
E |
|
|
|
E |
|
|
|
|
n-4 |
|
|
|
n-4 |
|
|
|
|
|
n = 5, 6, 7 |
|
only n = 7 |
|||
Naphth O2S |
|
|
|
|
|
Naphth O2S |
|||
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pd(OAc)2, PPh3 |
|
|
N |
||
N |
|
|
|||||||
|
|
|
|
|
|
||||
|
|
|
|
KOAc, MeCN, |
|
|
|
|
|
|
Br |
|
|
140 °C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
one of the products formed (yield not reported)
Scheme 22
IV.3.1 CASCADE CARBOPALLADATION: TERMINATION WITH ALKENES |
1381 |
The 3-exo-trig cyclization often wins over a 5-exo-trig process, even though both are possible in the second step as exemplified by the cyclization of a triene with two suitable terminators (Scheme 23).[34]
|
E |
PdLn |
E |
|
E |
I |
|
|
E |
73% |
|
|||
|
|
E = CO2Et |
|
|
|
|
Scheme 23 |
|
|
D.i.b. Insertion of Another Alkenyl Unit. In certain cases, however, the 3-exo-trig process may be retarded and an additional alkene moiety participates in the cascade carbopalladation. A pioneering example of this kind has been demonstrated by Overman and co-workers in their total synthesis of scopadulcic acid A, starting from an iodoalkenylsubstituted methylenecycloheptene derivative (Scheme 24). The first intramolecular carbopalladation occurs across the disubstituted double bond of the exomethylene group, and the trisubstituted endocyclic double bond acts as the terminator to give a tricyclic system, which was further elaborated to the natural product (Scheme 24).[35] It is remarkable that all three quaternary carbon centers can be created by intramolecular Heck reactions.
|
|
|
|
O |
|
|
|
|
O |
O |
I |
|
1. Pd(OAc)2, PPh3 |
|
|
|
|
||
|
H |
|
Ag2CO3, THF |
|
|
O |
|
reflux |
|
|
|
|
2. TBAF, THF |
|
|
OR |
|
|
|
|
82% |
HO |
||
|
|
|
|
|
|
OR = SiMe2 t-Bu |
|
|
H |
|
|
|
|
|
|
|
Scheme 24 |
||
The same tricyclic skeleton with an annelated benzene ring was prepared as a precursor to scopadulcic acid B (Scheme 25),[36] starting from an ortho-iodobenzoyl- substituted methylenecycloheptene derivative, and the o-iodobenzyl derivative with a shorter tether, led to the benzene-annelated lower homologue of the tricycle (Scheme 25).[37]
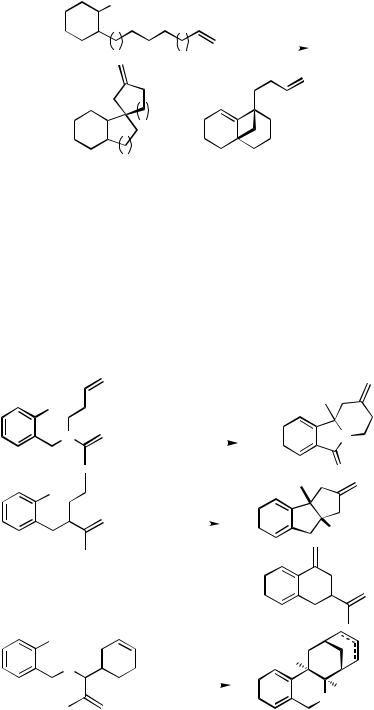
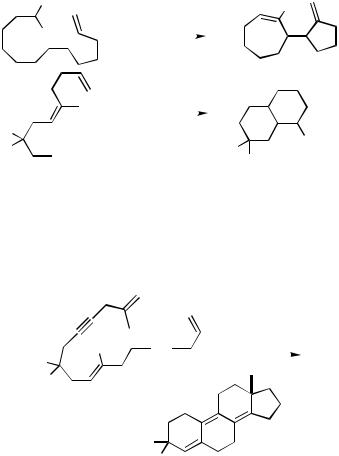
Three simpler ortho-substituted iodobenzene derivatives with two alkene moieties in the side chains have been cascade cyclized to give methylenespiroalkane-annelated indanes (Scheme 26)[18],[37] and bicyclic systems (Scheme 27).[38] This outcome clearly demonstrates that the intramolecular carbopalladations are determined by entropic factors, which always favor the formation of the smaller possible ring sizes.
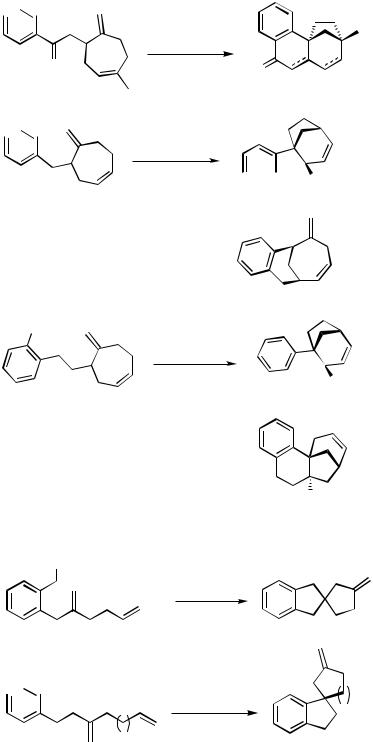
1382 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
 I
I
O

 I
I
I
Cl

 I
I
Pd(OAc)2, PPh3
Ag2CO3, THF
80 °C
80–85%
(10–14 g scale)
Pd(OAc)2, PPh3
Ag2CO3, MeCN
r.t., 2–8 h
+
Pd(OAc)2, PPh3
Ag2CO3, MeCN
83 °C, 10 h
90%
O |
|
8 |
|
13 |
7 |
14 |
|
||
|
|
|||
|
|
|
||
|
D13,14 + D7,8 |
|
||
 H 43%
H 43%
25%

 H
H
1.3 : 1
+
H
Scheme 25
Pd(PPh3)4,
Et3N, MeCN,
reflux, 5 h
57%
Pd(OAc)2, PPh3
Ag2CO3, MeCN
r.t. or 70 °C |
n |
|
n |
n = 1: 86% |
|
n = 2: 85% |
||
|
Scheme 26
IV.3.1 CASCADE CARBOPALLADATION: TERMINATION WITH ALKENES |
1383 |
||||||||||||||
|
|
|
|
|
|
OTf |
|
|
|
|
|
Pd(OAc)2, PPh3 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
Et3N, MeCN |
|
||
|
|
|
|
|
|
m |
|
|
n |
70 °C |
|
||||
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|||||
O |
|
|
|
|
|
|
|
|
|
|
|||||
|
|
n |
|
||||||||||||
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
m |
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
O |
|
|
|
O |
|
||||||||
|
|
|
m |
n |
% |
% |
|
|
|
||||||
1 |
|
1 |
72 |
0 |
|
|
|
||||||||
1 |
|
2 |
70 |
0 |
|
|
|
||||||||
2 |
|
1 |
53 |
5 |
|
|
|
||||||||
2 |
|
1 |
50 |
0 |
|
|
|
||||||||
Scheme 27
With the neopentyl relay in one of the side chains, the competition between fiveand seven-membered ring formation is clearly in favor of the five-membered ring to be formed while the discrimination between fiveand six-membered ring formation is not so clear-cut (Scheme 28).[37],[39] Tetracyclic systems are formed when the terminating double bond itself is in a ring (Scheme 28).[40]
|
I |
|
|
Pd(OAc)2, PPh3 |
|
|
|||
|
|
|
|
K2CO3, Et4NCl, MeCN |
|
|
|||
|
|
N |
|
|
80 °C, 3 h |
|
|
N |
|
|
|
|
|
||||||
|
|
|
92% |
|
|
|
|
||
O |
|
|
|
|
|
|
O |
||
|
I |
|
|
Pd(OAc)2, PPh3 |
|
|
|||
|
|
|
|
||||||
|
|
|
|
|
Ag2CO3, MeCN |
|
|
||
|
|
|
|
|
r.t., 2–8 h |
|
H |
||
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
||
|
|
|
81% |
|
|
|
|||
1.5 : 1
I |
|
Pd(OAc)2, PPh3 |
+ |
|
|
||
|
|
|
|||||
|
|
|
|
||||
|
|
PhOMe |
|
|
|
||
|
O |
130–140 |
°C, |
|
|
Me |
|
|
42 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
68% |
|
|
|
|
H |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
1:1.6 mixture of regioisomers
Scheme 28
1384 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
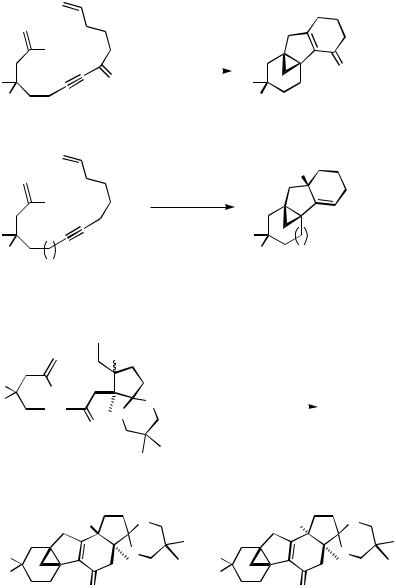
D.i.c. Alkyne Relay. The alkyne relay has frequently been used for zipper mode processes involving intramolecular carbopalladation reactions. Since the carbon – carbon triple bond possesses a higher reactivity than the C,C double bond, alkynyl groups are easily introduced into organic molecules with simple starting materials, and reactions across alkynyl groups occur with a high degree of stereoselectivity (because of their high predisposition for exo-dicyclizations), alkynyl groups are ideal relays.
The Pd-catalyzed intramolecular cascade cross-coupling of 1-halo-1,( 1)-dienynes [for 2-halo-1,( 1)-dienynes see Scheme 32] with a terminal double bond leads to 2-methylenecycloalkylidenecycloalkenes (Scheme 29),[10] whereas halodienynes with the initiating iodoalkenyl unit incorporated in the chain between the alkynyl relay and the alkenyl terminator yield methylenebicycloalkadienes (Scheme 29).[10]
|
|
SiMe3 |
|
Pd(PPh3)4 |
|
Me3Si |
|||||||||||||
|
|
|
|
|
|
|
|
Et3N, MeCN |
|
|
|
|
|
|
|
|
|||
|
|
I |
|
reflux, 5 h |
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
78% |
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Pd(PPh3)4 |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
Et3N, MeCN |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
I |
|
reflux, 6 h |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
76% |
|
|
|
|
|
|
|
SiMe3 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
SiMe3 |
|
E = CO2Et |
E |
||||||||||
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
E |
|||||||||||
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
Scheme 29 |
|
|
|
|
|
|
|
|
||
Even the tetracyclic steroid skeleton was successfully assembled in a zipper mode from an open-chain trienediyne with two alkynyl groups and a 2,2-disubstituted terminal double bond functioning as relays, as demonstrated by Negishi and co-workers (Scheme 30).[41]
|
|
|
Pd(PPh3)4 |
|
|
|
|
|
Et3N, MeCN |
|
|
I |
|
|
80 °C |
|
|
|
|
|
|||
|
|
||||
E |
|
|
|
||
76% |
|
|
|||
E |
|
|
|
||
E |
|
|
|
||
|
|
|
|||
|
|
|
|||
|
E |
E = CO2Et |
|
||
Scheme 30
Termination by 2,2-disubstituted alkenyl groups can lead to bicyclo[n.1.0]alkane derivatives if the starting unit is an ortho-substituted iodoaryl or a 1-haloalkenyl moiety
(Scheme 31).[10],[21],[42]

|
IV.3.1 |
CASCADE CARBOPALLADATION: TERMINATION WITH ALKENES |
1385 |
|||||||
|
|
|
|
|
|
|
|
|
|
E |
|
I |
|
|
Pd(OAc)2, PPh3 |
|
|
E |
|||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
Et4NCl, HCO2Na |
|
|
|
|
|
N |
|
|
DMF, 70 °C, 1 h |
|
|
|
|||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|||
|
Ac |
E |
65% |
|
|
N |
|
|||
|
|
|
|
E |
|
|
|
|
Ac |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I |
|
Pd(PPh3)4, Et3N |
|
|
|
||
|
|
|
|
|
MeCN, reflux, 12 h |
|
|
|
||
E |
|
|
|
|
|
|
|
|||
|
56% |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
||
E |
|
|
|
|
E = CO2Et |
E |
|
|||
|
|
|
|
|||||||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
E |
|
|||
Scheme 31
However, a halodienyne with a 2-haloalkenyl moiety as a starter and a 2,2-disubsti- tuted terminal double bond yields a tetracyclic system with the three-membered ring bridging the bond common to the A- and B-rings (Scheme 32).[43]
|
|
|
|
|
|
|
|
R1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pd(PPh3)4, |
R2 |
||
|
|
Br |
|
|
|
Ag2CO3, MeCN |
|||
|
|
|
|
|
|||||
|
|
|
|
|
80−130 |
°C, 3 h to 3 d |
R2 |
||
|
|
|
|
|
|
||||
O |
|
|
R1 |
|
|
O |
|||
|
|
|
|
||||||
|
|
|
|
|
R2 |
|
R1 = Me; R2 = H: |
62% |
|
|
|
|
|
|
R2 |
|
|||
|
|
|
|
|
|
R1, R2 = CO2Me: |
71% |
||
|
|
|
|
|
|
|
|
||
Scheme 32
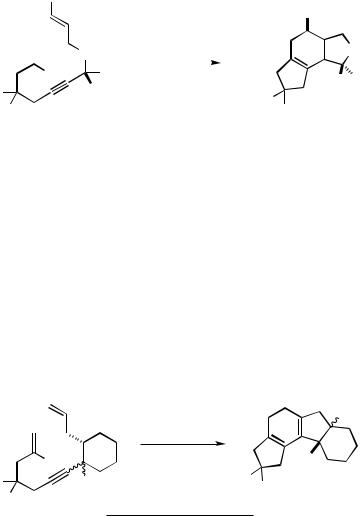
The same tetracyclization mode is observed for any 2-halodienyne in which the two tethers between the 2-haloethenyl starter and the alkynyl relay as well as between the latter and the terminal double bond are four or more atoms long, even when the alkenyl terminator is not substituted in the 2-position (Scheme 33, Eqs. 1 and 2).[30] Thus, a cyclopentanone derivative fitted with a 3-ethenyl and a 2-(8 -bromo-2 -oxonon- 8 -en-3 - ynyl) tether did not cyclize to the tetracyclic steroid skeleton, but to the pentacycle with a five-membered B-ring and a bridging cyclopropane moiety, as was proved by an X-ray crystal structure analysis of the cis-diastereomer (Scheme 33, Eq. 3).[30]
2-Halo-1,( 1)-dienynes, in which at least one of the tethers between the multiple bonds is only three atoms long, generally undergo a two-stage intramolecular cascade carbopalladation terminated by dehydropalladation to yield a bicyclic 1,3,5-hexatriene, which, under the same conditions, cyclizes to a tricyclic system with a central cyclohexadiene. The yields are best when both rings formed by intramolecular cross-coupling are five-membered, and they are consistently better when the first formed ring is fivemembered. With a substituent at the olefinic terminus, the final 6 -electrocyclization apparently proceeds with a high degree of rotaselectivity, as the terminal substituent ends up trans with respect to the former propargylic substituent that stands finally in the other ring. Starting with a bromodienyne tethered to a ring, a tetracyclic system is produced by this cascade (Scheme 34).[44]–[47]
1386 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
|
|
|
|
Pd(OAc)2 (10 mol%), |
|
|
|
PPh3 (20 mol %), |
|
|
Br |
Ag2CO3 (3 equiv.), |
(1) |
|
|
MeCN, 80 °C, 3 d |
|
|
O |
|
O |
|
E |
45% |
|||
E |
||||
E |
|
|
E |
|
E = CO2Et |
|
|
single product |
|
|
|
|
|
|
|
|
|
|
H |
|
|
Br |
|
|
|
as above |
|
|||||
|
|
|
|
|
|
|
|
||||
E |
|
|
|
|
|
|
|
|
|
E |
n–5 |
|
|
|
|
|
|
n |
Yield (%) |
||||
E |
|
|
|
|
|
|
|||||
|
n–5 |
|
|
|
|
|
E |
|
|||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
6 |
74 |
single prod. |
|
|||
E = CO2Et |
7 |
76 |
single prod. |
|
|||||||
|
|
|
|
|
8 |
30 |
|
|
|
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
E |
|
|
|
|
|
|
|
|
Pd(OAc)2, PPh3 |
|
|
|
Br |
|
|
|
|
|
Ag2CO3, (i-Pr)2NH |
|
|||
E |
|
|
|
O |
|
MeCN, 120 °C, 15 h |
|
||||
|
|
|
O |
O |
|
40% |
|
||||
|
|
|
|
|
|||||||
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
||
|
cis/trans = 1 : 3.5 |
|
|
|
|
|
E = CO2Et |
|
|||
|
|
|
H |
|
|
O |
|
|
H |
O |
|
E |
|
|
|
|
|
O |
|
+ |
|
|
O |
|
|
|
|
|
|
E |
|||||
E |
|
|
|
|
|
|
|
E |
|
||
|
|
|
O |
|
oil |
|
|
|
O |
crystals |
|
|
|
|
|
|
|
|
|
|
|
||
Scheme 33
(2)
(3)
Nitrogenand oxygen-containing tricyclic dienes can be prepared from heteroatomcontaining and appropriately (on nitrogen) substituted acyclic 2-bromodienynes without problems. Thus, a number of diazaand dioxatricycles were obtained in moderate to good yields from the corresponding precursors, respectively (Scheme 35).[48] Surprisingly, a diazadibromodienyne underwent tricyclization to the same product as the diazamonobromodienyne. This transformation involves a reduction analogous to the one in the Pd-catalyzed homocoupling of aryl halides (see above).
IV.3.1 CASCADE CARBOPALLADATION: TERMINATION WITH ALKENES |
1387 |
R3
|
|
|
|
|
|
|
|
|
|
R3 |
|
|
|
|
|
|
Br |
X |
R1 |
|
|
conditions |
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
R |
2 R1 |
|||||
|
|
|
|
|
|
|
|
|
|||||
E |
R |
2 |
|
E = CO2Et |
|
|
|
|
|||||
|
|
E |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|||||||
E |
|
|
|
|
|
E |
|
|
|||||
|
|
|
|
|
|
|
|
||||||
|
X |
R1 |
R2 |
R3 |
Conditionsa |
Yield (%) |
|
|
|||||
|
CMe2 |
H |
OMe |
H |
A |
60 |
|
|
|||||
|
C(CH2)5 |
|
|
OMe |
H |
A |
87 |
|
|
||||
|
CMe2 |
H |
OMe |
H |
A |
60 |
|
|
|||||
|
CMe2 |
H |
OAllyl |
H |
B |
58b |
|
|
|||||
|
O |
H |
OMe |
H |
C |
84 |
|
|
|||||
|
O |
H |
OEt |
H |
D |
81 |
|
|
|||||
|
O |
H |
Oi-Pr |
H |
E |
95 |
|
|
|||||
|
O |
H |
OPh |
H |
F |
51 |
|
|
|||||
|
O |
H |
OPMB |
H |
G |
25 |
|
|
|||||
|
CH2 |
H |
OMe |
Ph |
H |
83 |
|
|
|||||
aA: Pd(OAc)2, PPh3, Ag2CO3, MeCN, 80 °C, 36 h. – B: As in A, but 60 °C, 6 h. – C: As in A, but 9 h. – D: As in A, but 6 h. – E: As in A, but 60 °C, 4 h. – F: As in A, but 12 h. – G: As in A, but 110 °C,
7 h. – H: Pd(OAc)2, PPh3, K2CO3, MeCN, 110 °C, 3 d.
bA dihydroindenotetrahydrofuran (23%) was also isolated.
|
Pd(OAc)2, PPh3 |
H |
|
|
Ag2CO3, MeCN |
|
|
|
80 °C, 3 h |
|
|
|
Br |
RO |
|
|
|
||
E |
OR |
E |
|
E |
|||
|
|
E
OR Yield (%)
OMe cis (trans) 48 (88)
OH cis (trans) 80 (85)
Scheme 34
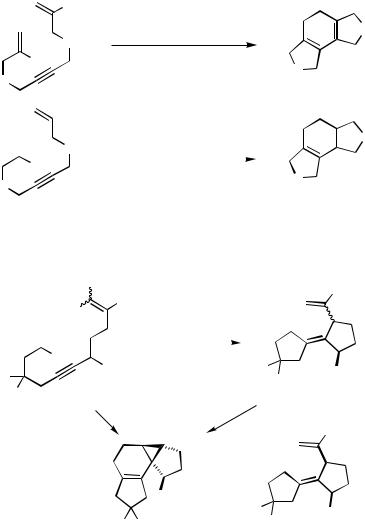
Another potentially powerful sequence arises by combining one or two intramolecular Heck-type couplings with an intraor intermolecular Diels–Alder addition (for early examples of inter–intermolecular one-pot domino Heck–Diels–Alder reactions see Refs. [49] and [50]). An all-intramolecular version of such a sequence has been shown to proceed reasonably smoothly for terminally alkoxycarbonyl-substituted 2-bromotrideca- 1,11-dien-6-ynes under palladium catalysis at 130 °C. At 80 °C, the sequential reaction stops after the two consecutive Heck-type cyclizations and subsequent -hydride elimination to give a 1,3,6-triene; apparently only the (E )-isomer undergoes the intramolecular Diels – Alder reaction, as the (Z )-1,3,6-triene is observed accompanying the tetracyclic system obtained at 130 °C (Scheme 36).
1388 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATION |
X
Pd-cyclea
° NTs
NTs
K2CO3, MeCN, 80 C, 48 h
Br |
X = H: 55% |
|
|
X = Br: 60 % |
TsN |
||
|
|||
|
|
TsN
|
|
|
Pd-cyclea |
|
O |
|
|
O |
K2CO3, MeCN, 80 °C, 48 h |
|
|
|
|
|
|
||
|
|
Br |
> 80 % |
O |
|
O |
|
||||
|
|
|
|||
aPd-cycle = palladacycle (1a in Section IV.1) prepared from Pd(OAc)2 and P(o-Tol)3
|
|
|
|
|
Scheme 35 |
|
|
|
|
|
||
|
|
|
|
Me |
|
|
|
|
|
|
|
CO2Me |
|
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
MeO2C |
|
Pd(OAc)2, PPh3 |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
Ag2CO3, MeCN |
|
|
|
|
|
|
|
|
|
|
|
|
80 °C, 8 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
Br |
OMe |
76% |
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|||
E |
|
|
|
|
|
|
|
|
|
MeO |
||
|
|
|
|
|
|
|
E |
|||||
E |
(E/Z) = 1.67:1 |
|
|
|
(E/Z) = 1.67:1 |
|||||||
|
|
|
|
78% |
|
|
|
|
|
|
|
CO2Me |
Pd(OAc)2, PPh3 |
|
|
|
|
|
|
|
|
||||
Ag2CO3, MeCN |
|
|
|
|
|
|
|
|
|
|||
130 |
°C, 14 h |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
OMe |
|
|
|
|
|
||
|
|
|
|
|
|
|
E |
|
|
|
|
MeO |
E = CO2Et |
E |
E |
E |
|||||||||
|
|
|
|
|
||||||||
|
|
|
|
|
(47%) |
|
(31%) |
|||||
Scheme 36
The cycloisomerization reaction of dienand oligoenynes has been developed by Trost and Shi and shows its remarkable potential by the palladium zipper, which has been used to create seven carbocyclic rings in one step (Scheme 37).[28]
Cycloisomerization reactions of dienynes having methylenecyclopropane moieties with tetrasubstituted double bonds, which are readily accessible by Pd-catalyzed substitution on 1-propenylcyclopropyl tosylate or chloride,[51] give rise to the formation of cyclopropane-ring-opened products. This reaction most probably does not proceed by simple (n 1)-exo-trig cyclization to give a cyclopropylpalladium intermediate, but a sequence of (n 1)-exo-trig and 3-exo-trig cyclizations, followed by a cyclopropylcarbinyl to homoallyl rearrangement of an intermediate spiropentylmethylpalladium compound,
