

IV.10.3 Other Reactions Involving
Palladacyclopropanes and
Palladacyclopropenes
ARMIN DE MEIJERE and OLIVER REISER
Although palladacyclopropanes and palladacyclopropenes can be postulated as intermediates when palladium in the oxidation state 0 or 2 interacts with an alkene or an alkyne, there is very little evidence that Pd-catalyzed reactions of alkenes and alkynes actually proceed this way.
In principle, a palladacyclopropane might be generated starting from palladium(0) or palladium(II) 1 and an alkene 2 (Scheme 1): after coordination to form a -complex 3, oxidative addition would yield a -complex 4, a pallada(II)- or pallada(IV)cyclopropane. Likewise, the analogous reaction with an alkyne 5 would lead to a palladacyclopropene 7.
0 or +2 |
|
|
|
0 or +2 |
|
+2 or +4 |
||||
Pd |
+ |
|
|
Pd |
|
Pd |
||||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
1 |
2 |
3 |
|
4 |
||||||
0 or +2 |
|
|
|
0 or +2 |
|
+2 or +4 |
||||
Pd |
+ |
|
|
Pd |
|
Pd |
||||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
1 |
5 |
6 |
|
7 |
||||||
|
|
Scheme 1 |
|
|
|
|
||||
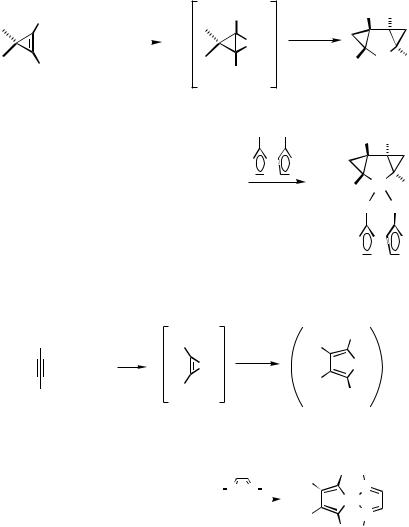
Conclusive evidence for palladacyclopentanes[1] and palladacyclopentadienes[2],[3] as potentially stable intermediates has been put forward, and they have a close analogy to such metallacycles with other metals.[4],[5] They frequently are intermediates in metalcatalyzed or -mediated formal [2 2 2] cyclotrimerizations of alkynes and strained alkenes. It is plausible to assume that palladacyclopropanes or -cyclopropenes also play a role in such formal [2 2 2] processes catalyzed by palladium(0), for example, in the type of Pd-catalyzed cyclotrimerization of 3,3-dimethylcyclopropene reported by Binger et al.[6] (see also Sect. IV.2.4), for which a palladacyclopentane as intermediate was already isolated.[7] Moreover, dimethyl 3,3-dimethylcyclopropenedicarboxylate 8 has recently been shown to form the tricyclic palladacyclopentane derivative 10, the structure
Handbook of Organopalladium Chemistry for Organic Synthesis, Edited by Ei-ichi Negishi ISBN 0-471-31506-0 © 2002 John Wiley & Sons, Inc.
1647
1648 |
IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATIONS |
of which was proved by X-ray analysis of its 1,1 -bis(diphenylphosphinyl)ferrocene adduct 11 (Scheme 2).[1] It is most reasonable that 10 is formed via carbopalladation of 8 by the palladacyclopropane derivative 9.
Pd(dba)2 reacts rapidly with dimethyl acetylenedicarboxylate (DMAD) to form the polymeric palladacyclopentadiene TCPC(13),[2] which could be fully characterized as diazadiene complexes 14 (Scheme 3).[3]
The palladacyclopropene 12 would most probably be an intermediate en route to 13, and, indeed, 1:1 complexes of palladium and DMAD have been obtained and characterized by 1H NMR and IR spectroscopy with stabilizing ligands such as triphenylphosphine[2] and N,N -di-tert-butyl-1,4-diaza-1,3-diene[3] present (Scheme 4). Unfortunately, however, it was
E
[Pd |
(dba) |
] |
CHCl |
2 |
3 |
|
3 |
E
8
E
+ Pd(dba)
2
E
E = COMe
2
E
8
Pd
E
9
PPh2 PPh2
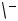
 Fe
Fe
Scheme 2
E E
DMAD
Pd
E
E
E E
E Pd E
10
E E
E |
Pd |
E |
|
|
|
Ph2P |
|
PPh2 |
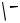
 Fe
Fe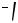
11
E
Pd
E
n
12 |
|
13 |
|
|
|
|
|
E |
R |
|
R N |
|
E |
N |
|
N R |
|||
|
|
|
Pd |
|
|
|
|
||
|
|
|
E |
N |
|
|
|
|
|
|
|
|
E |
R |
|
|
14 |
|
|
Scheme 3 |
|
|
|
|
IV.10.3 REACTIONS OF PALLADACYCLOPROPANES AND PALLADACYCLOPROPENES |
1649 |
|||||||||||||||||||
|
E |
E |
PPh3 |
|
|
E |
|
PPh3 |
|
|||||||||||
|
|
|
|
|
|
|
2 PPh3 |
|
or |
|
|
|
|
|||||||
|
|
|
+ Pd(dba)2 |
|
Pd |
|
|
|
Pd |
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
E |
PPh3 |
|
|
|
|
|
PPh3 |
|
|
E |
|
|
|
E |
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
|
E = CO2Me |
|
15 |
|
|
|
|
16 |
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
E |
t-Bu |
|
|
E |
|
t-Bu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
N |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
or |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
Pd |
|
|
|
|
Pd |
|
|
|
|
|
|
t-Bu |
|
N N |
|
t-Bu |
E |
N |
|
|
|
|
|
N |
|
|||
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
t-Bu |
|
|
E |
|
t-Bu |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
17 |
|
|
|
|
18 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 4 |
|
|
|
|
|
|
|
|
not possible to distinguish whether their structures are best described as -complexes 15 and 17 or as palladacyclopropenes 16 and 18, respectively, but the latter seem plausible by analogy to platinacyclopropenes being well established via similar pathways.[8]
In enyne cycloisomerizations developed by Trost (see Sect. IV.2.5 and IV.3.1), palladacycles are also likely intermediates (Scheme 5).[9] In the palladium(II)-initiated cyclization of an enyne 19, the palladacyclopentene 21 has been postulated, requiring palladium to adopt the formal oxidation state 4. Catalytic cycles involving Pd(II) – Pd(IV) are not well precedented; however, by now several reactions have been reported in which Pd(IV) intermediates are most likely,[10] – [16] and one palladium(IV)
H
|
|
|
|
|
|
4+ |
|
H |
|
|
|
|
|
|
|
|
Pd |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
20 |
|
|
4+ |
|||
|
|
Pd2+ |
|
|
|||||
|
|
|
|
|
Pd |
||||
|
|
|
|
|
|
|
|
21 |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
||
19 |
|
|
|
|
|
|
|||
|
|
|
|
|
|
||||
|
|
|
|
|
|
H |
|||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
Pd2+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22 |
|
|
|
|
||
|
|
|
|
|
|
|
|||
4+
 Pd H
Pd H
23 |
24 |
Scheme 5
1650 IV Pd-CATALYZED REACTIONS INVOLVING CARBOPALLADATIONS
complex has been fully characterized by an X-ray crystal structure analysis.[17] Most probably, 21 would be formed from the -complex 22 via the palladacyclopropene 20 by intramolecular carbopalladation of the double bond.
REFERENCES
[1]A. S. K. Hashmi, F. Naumann, R. Probst, and J. W. Bats, Angew. Chem. Int. Ed. Engl., 1997, 34, 104.
[2]K. Moseley and P. M. Maitlis, J. Chem. Soc. Dalton Trans., 1974, 169.
[3]H. tom Dieck, C. Munz, and C. Müller, J. Organomet. Chem., 1990, 384, 243.
[4]E. Negishi, Acc. Chem. Res., 1987, 20, 65–78.
[5]N. E. Schore, Chem. Rev., 1988, 88, 1081–1119.
[6]P. Binger, J. McMeeking, and U. Schuchardt, Chem. Ber., 1980, 113, 2372.
[7]P. Binger, H. M. Bück, R. Benn, and R. Mynott, Angew. Chem. Int. Ed. Engl., 1982, 21, 62.
[8]G. B. Young, in Comprehensive Organometallic Chemistry II: A Review of the Literature 1982–1994, Vol. 9, E. W. Abel, F. G. A. Stone, and G. Wilkinson, Eds., Pergamon Press, Oxford, 1995, 533–588.
[9]B. M. Trost, Acc. Chem. Res., 1990, 23, 34–42.
[10]A. J. Canty, Acc. Chem. Res., 1992, 25, 83–90.
[11]M. Catellani, G. P. Chiusoli, and C. Castagnoli, J. Organomet. Chem., 1991, 407, C30.
[12]O. Reiser, M. Weber, and A. de Meijere, Angew. Chem. Int. Ed. Engl., 1989, 28, 1037.
[13]K. Albrecht, O. Reiser, M. Weber, B. Knieriem, and A. de Meijere, Tetrahedron, 1994, 50, 383.
[14]T. Ito, H. Tsuchiya, and A. Yamamoto, Bull. Chem. Soc. Jpn., 1977, 50, 1319.
[15]A. Moravskiy and J. K. Stille, J. Am. Chem. Soc., 1981, 102, 4182.
[16]M. K. Loar and J. K. Stille, J. Am. Chem. Soc., 1981, 103, 4174.
[17]M. Catellani and M. C. Fagnola, Angew. Chem. Int. Ed. Engl., 1995, 33, 2421.
