
Revision Sinus Surgery
.pdf
302 |
Seth J. Kanowitz, Joseph B. Jacobs, and Richard A. Lebowitz |
frontoethmoidectomy procedures followed by stenting of the FSOT with a wide variety of rigid or soft materials. Initially a gold tube, placed endonasally, was utilized by Ingals to stent the region of the FSOT until it was mucosalized [13]. In 1921, Lynch placed a firm rubber tube in the FSOT in 15 patients until postoperative day five. After 2.5 years of follow-up he reported a 100% success rate [15]. However, subsequent studies demonstrated a long-term failure rate of approximately 30% [17, 18].
The relative success of these early novel techniques led others to experiment with different stent materials in the 1940s and 1950s. Goodale, Harris, and Scharfe described
34 their experiences with the use of tantalum, an inert basic element originally discovered by Eckenberg in 1802, for frontal sinus stenting [7, 8, 10, 22]. Goodale employed the material as a thin sheet sutured to the orbital periosteum during revision external frontoethmoidectomies as a way of minimizing circumferential granulation tissue and scarring [8, 9]. Extending on Goodale’s work, Harris and Scharfe described their limited series of patients where tantalum tubes were placed from the frontal sinus into the nasal cavity [10, 22]. In all cases, patients demonstrated resolution of their symptoms and patency of their frontal sinus. Each author noted decreased scarring of the FSOT, improved epithelialization, and decreased granulation tissue formation. However, it should be noted that during the time of these procedures antibiotics were employed for the first time, and thus are probably partially responsible for the improved outcomes. Other materials, such as Dacron woven arterial graft have also been employed for similar indications and with similar results. However, Barton employed this material during a Lynch external frontal sinusotomy, for permanent stenting of the FSOT over a 17-year period in 34 patients [2]. No stents were removed and all patients were relieved of their frontal headache symptoms.
In contrast to today’s modern soft materials, the original designs involved rigid materials likely employed out of convenience and limited by current technological standards. However, in 1976 Neel demonstrated the superiority of thin, pliable Silastic sheeting for the first time in a canine model [17]. In those treated with firm rubber stents, significant fibrosis and osteoblastic activity with little or no epithelialization resulted. In contrast, normal mucosal lining was observed on histological specimens in those ducts stented with thin Silastic sheeting. He attributed the difference to local ischemia, impaired drainage, and localized infection around the rigid tubes. He subsequently reviewed a series of patients after an average of 13.5 years of follow-up and reported a 29% failure rate with rubber tubing and a 17% failure rate with thin Silastic sheeting [18].
With the dawn of the endoscopic era, Schaefer and Close extended upon the ideas initially proposed by Neel
and employed Silastic tubing for small endoscopic frontal sinusotomies (4–6 mm) in four patients [21]. However, a 50% stenosis rate resulted, which was attributed to a failure to maintain a postoperative communication between an air passage and the mucosa, thus resulting in massively hypertrophied mucosa and obstruction of the frontal sinus ostium. Since then, numerous authors have reported their experience with the external or endoscopic placement of Silicone tubes or rolled sheeting to help maintain patency of the FSOT (Fig. 34.1) [1, 3, 5, 6, 9, 11, 12, 14, 16, 20, 23–25].
In cases where an external approach is still necessary, there is some data supporting the role of frontal sinus stenting, although none of the reports are compared against nonstented controls. In a comprehensive review of 164 cases, Amble placed thin Silicone rubber sheeting to reconstruct the FSOT after a modified external Lynch procedure in which the frontal process of the superior maxilla was preserved [1]. Of the 164 patients studied, 79 of the patients had previous failed sinus surgical procedures addressing the frontal sinus and thus were undergoing a revision. After FSOT reconstruction with Silicone sheeting for 6–8 weeks, 96% achieved resolution of their symptoms at a mean follow-up of four years. Only 18% of the patients required a revision procedure to achieve 4 years during the study period.
Yamabosa also reported on his experience in placing a silicone T-tube in the FSOT via a Lynch incision in 18 patients presenting with frontoethmoid mucoceles [25]. Three of these patients had failed previous external frontal procedures, and all patients failed a transnasal approach at widening the FSOT due to thick bone formation. Removal of the tube was determined by aeration of the frontal sinus on CT imaging and lack of polypoid mucosa or mucous discharge on flexible endoscopic exam. Although the exact length of follow-up is unclear, 16 patients achieved patency of the FSOT and resolution of their symptoms. The two failures developed scar tissue and polyps of the FSOT at 3 and 5 months after stent removal.
As the endoscopic era began, more authors started to report their experience with endoscopic frontal sinus stenting. In a limited trial, Schaefer and Close endoscopically placed thin Silastic tubing as a frontal sinus stent in patients with small frontal sinusotomies (4–6 mm), resulting in a 50% failure rate in the four patients studied [21]. Weber retrospectively reviewed 12 patients who underwent various endoscopic Draf-type procedures for frontal sinusotomy followed by a 6-month course of FSOT stenting with either a Rains self-retaining Silicone tube, U-shaped Silicone tube, or an H-shaped Silicone tube [23]. While all patients had improvement or resolution of their headache symptom, only six FSOTs were endoscopically visible at a mean of 19.4 months of followup. Five other FSOTs were deemed functionally patent
Stenting in Revision Sinus Surgery
due to evidence of aeration on imaging studies. Hoyt reported similar results in 21 patients (32 stents) who had vented tubular plastic stents placed endoscopically over a guidewire for an average of 8 weeks [12].
Using the stent that now bears his name, Freeman placed a biflanged Silicone tube in an external or endoscopic fashion in 64 sinuses [19]. The Freeman frontal sinus stent is unique in that it requires an introducer and the distal flange is contained within a dissolving gel cap to allow for easier insertion. After a mean follow-up of 29 months, six sinuses eventually required fat obliteration due to return of polyposis or restenosis of the FSOT. Similarly, Rains endoscopically placed 102 stents (soft Silicone tube with a tapered collapsible bulb) in 67 patients for an average duration of 35 days [20]. After a follow-up of 8– 48 months, a 94% endoscopic patency rate was reported, with allergic fungal sinusitis present in all failures.
Preoperative Assessment
■Carefully review the sinus anatomy on CT to determine the potential surgical diameter of the frontal sinus neo-ostium, as limited by the frontal beak, anterior skull base, medial orbit, and cribriform plate.
303
■Evaluate the preoperative CT for radiographic evidence of extensive sinonasal polyposis, allergic fungal sinusitis, and/or osteitis of the bone of the FSOT.
■Evaluate the preoperative CT for evidence of lateralization or scarring of the middle turbinate or middle turbinate remnant.
■Examine the FSOT with angled nasal endoscopes to determine the presence of polyposis or hyperplastic mucosa in the frontal recess, scarring or synechiae from prior surgery, and previous partial middle turbinectomy with lateralization of the remnant fragments.
Indications for Stenting
While many non-case-controlled reports of FSOT stenting demonstrating successful patency rates exist in the literature, no standardized indications exist to guide surgeons in the implementation of these devices.
■Routine stenting in uncomplicated cases is not recommended, and thus FSOT stents should be employed on a case-by-case basis based upon the surgeon’s assessment of the patient’s relative risk of postoperative FSOT stenosis.
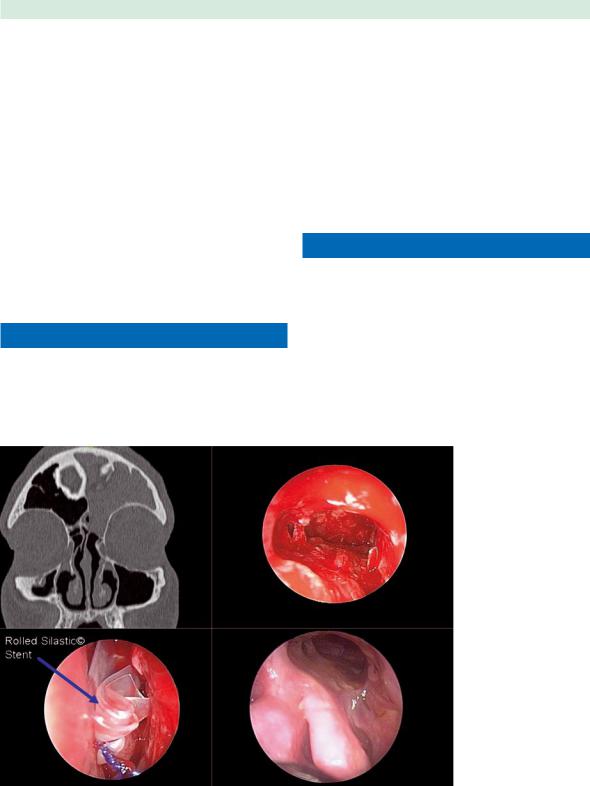
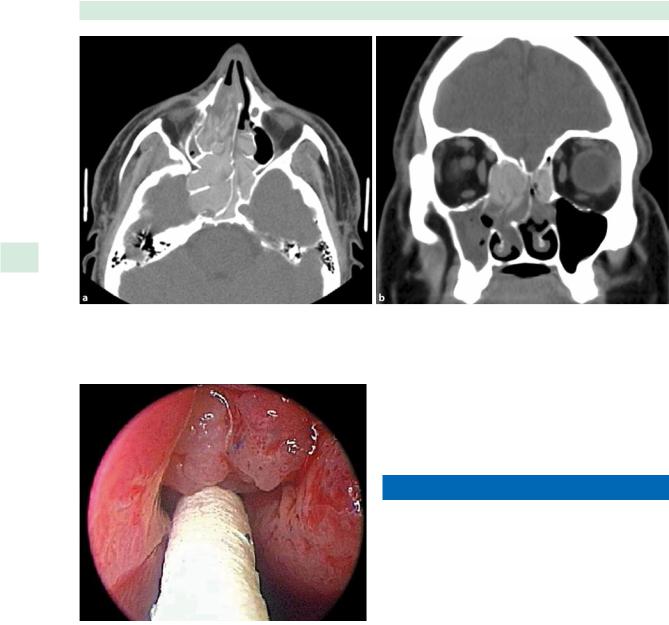
Fig. 34.1 Chronic left frontal sinusitis (left upper picture) requiring revision endoscopic frontal sinusotomy (right upper picture). A rolled Silastic stent (left lower picture) was placed in the frontal recess until mucosalization was complete (right lower picture)
|
|
304 |
Seth J. Kanowitz, Joseph B. Jacobs, and Richard A. Lebowitz |
||
|
|
Several intrinsic and extrinsic conditions need to be con- |
5. Destabilized or lateralized middle turbinate or |
||
|
|
sidered as risk factors for FSOT stenosis, and thus, as po- |
|
middle turbinate remnant. |
|
|
|
tential relative indications for stenting. |
|
6. |
Draf III or tumor resection procedures with muco- |
|
|
In one review, Hosemann demonstrated a doubling |
|
sal trauma and extensive bone exposure. |
|
|
|
(16% vs. 33%) of the rate of FSOT stenosis when the in- |
7. |
Frontoethmoid mucoceles, which usually indicate |
|
|
|
traoperative diameter of the neo-ostium was less than |
|
long-standing chronic sinus disease or previous |
|
|
|
5 mm. [11]. This is similar to the indications (4–6 mm) |
|
surgical failures. |
|
|
|
employed by Schafer and Close [21]. Therefore, a FSOT |
|
|
|
|
|
diameter of less than 5 mm at the completion of the fron- |
|
|
|
|
|
tal sinusotomy is often considered a relative indication |
|
|
|
|
|
Duration of Stenting |
|||
|
|
for stenting. Other relative indications for FSOT stenting |
|||
|
|
|
|
||
|
|
during revision frontal sinus surgery are listed below. |
Similar to the indications for stenting, no prospective |
||
34 |
|
■ Relative indications for FSOT stenting: |
|
case-controlled studies have been performed to investi- |
|
|
|
|
gate the optimal duration of FSOT stenting. While most |
||
|
|
1. Frontal sinus neo-ostium diameter less than 5 mm. |
authors recommend a temporary period of stenting, |
||
|
|
2. Extensive or circumferential exposure of bone in |
Barton employed his Dacron woven arterial graft per- |
||
|
|
the FSOT, which may lead to long-term postopera- |
manently over 17 years [2]. Whenever stent removal is |
||
|
|
tive crusting. |
|
deemed appropriate, all current authors report successful |
|
|
|
3. Sinonasal polyposis or hyperplastic mucosa, which |
removal of the stenting material in the office using endo- |
||
|
|
may lead to difficulty with postoperative endo- |
scopes and endoscopic sinus instrumentation. |
||
|
|
scopic visualization and debridement |
|
The 6-week time frame that is often discussed comes |
|
4.Evidence of osteitic bone or osteo-neogenesis visufrom the canine model by Neel where he demonstrated alized on CT imaging or encountered intraoperahistologically that re-epithelialization of the FSOT
tively (Fig. 34.2). |
stented with thin Silicone rubber is complete within |
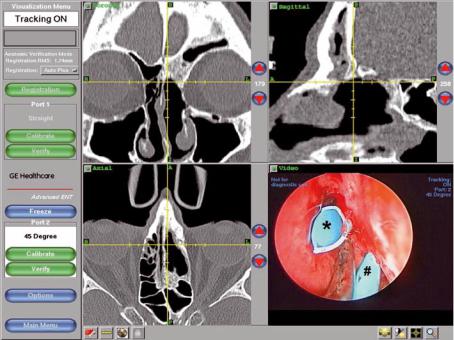
Fig. 34.2 Intraoperative surgical navigation with probe in stenotic left frontal sinus outflow tract during revision endoscopic frontal sinusotomy. The area of neo-osteogenesis has been removed by high-speed angled diamond burr and a Parrell frontal sinus stent (Medtronic) placed in the FSOT (*) as well as a large supraorbital ethmoid cell (#)

Stenting in Revision Sinus Surgery
approximately 8 weeks [17]. He subsequently employed these findings to his patient population and removed Silastic sheeting stents beginning at a minimum of 6 weeks postoperatively (mean 6 months). After a 7-year followup period he reported an 80% success rate [18].
In many of the trials already outlined above, average stenting time with soft Silicone stents ranges from 4 to 8 weeks with a success rate of 80–90% [1, 3, 6, 13, 20, 21]. While the length of follow-up and definition of success or patency varies among authors, all arrive at similar conclusions. However, Weber recommended removal of the various stents he employed in revision endonasal frontal sinusotomy at 6 months and cited improved patency with a longer duration of stenting [23]. The 6-month duration was adapted from Montgomery tubes inserted during tracheal surgery, as it was felt many months were necessary to allow for stabilization of the subepithelial scar. Freeman also described a period of stenting after revision frontal sinus surgery lasting between 6 and 12 months for patients stented to correct FSOT stenosis [6]. However, the duration of stenting was once again determined arbitrarily.
More recently, Dubin and Kuhn reported their experience with the management of the FSOT after open and endoscopic procedures for the removal of frontal sinus osteomas [5]. Five of 12 patients (3 open, 2 endoscopic) were stented with rolled Silastic sheeting (0.01 inches, or 0.25 mm thick) for an average of 9.2 months (range 6–17 months). One patient developed a scarred frontal recess despite 12 months of stenting after an osteoplastic flap approach. Casiano employed similar methods (1- mm-thick rolled Silastic sheeting) to stent the common frontal sinus ostia after endoscopic modified Lothrop procedures [24]. The stent was removed in the office at the 2-month postoperative visit. A patency rate of 60% was achieved, while 32% remained stenotic, and symptom improvement was achieved in 72% at a mean fol- low-up of 22 months (range 6–75 months). However, it is important to note that these results did not differ sta-
305
tistically from a comparison group of nonstented patients undergoing the same Draf III procedure.
Temporary short-term stenting may also play a role in revision frontal sinus surgery. In cases of extensive sinonasal polyposis, hyperplastic mucosa, or after technically demanding FSOT dissections where the mucosa is inevitably traumatized and bone exposed, placement of a soft Silastic stent may aid limiting the amount of postoperative crusting and/or endoscopic identification of the FSOT during postoperative endoscopy. The presence of a stent inherently limits the amount of FSOT probing necessary to debride the operative cavity. Removal of the stent at the first or second postoperative visit when the operative cavity is clean and mucosal inflammation controlled may enhance long-term results.
Surgical Technique
■Most commercially available frontal sinus stents can be easily placed via an endoscopic approach.
■Inadvertent displacement of mucosal flaps into the frontal sinus itself during stent placement should be avoided.
After creation of a frontal sinusotomy, the stent is grasped at the end with the flanges using a 90 side-to-side giraffe forceps (Fig. 34.3). The stent is then introduced into the FSOT under endoscopic visualization with a 70 rigid endoscope. The forceps are released and the instrument is gently maneuvered out of the nose. The stent can then be manipulated with an angled frontal sinus seeker. The stent should move freely within the FSOT and the flanges should be deployed beyond the internal frontal sinus ostium. In addition, the mucosa of the FSOT should lay flush around the stent and inadvertent displacement of mucosal flaps into the frontal sinus itself should be avoided. If the stent does not meet these requirements, then it should be removed and replaced properly. The stent should then
Fig. 34.3 90-degree side-to-side giraffe forceps grasping a Parrell frontal sinus stent (Medtronic, Minneapolis, MN, USA). The flanges are grasped within the forceps to allow for easier endoscopic placement
306 |
Seth J. Kanowitz, Joseph B. Jacobs, and Richard A. Lebowitz |
34 |
Fig. 34.4 Endoscopic view of a Parrell frontal sinus stent |
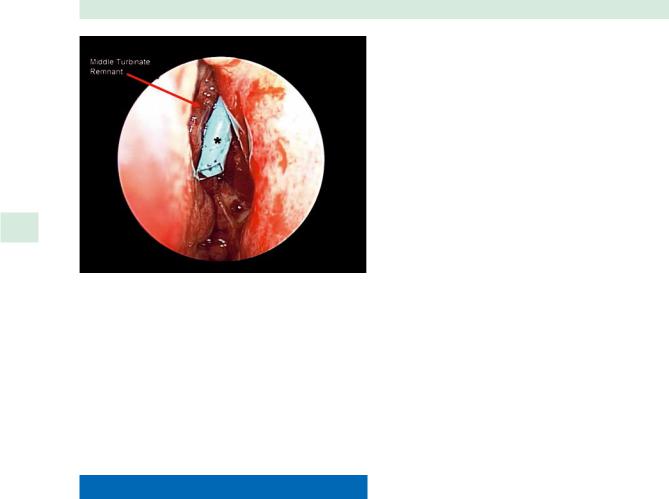
(*; Medtronic) in the left frontal sinus outflow tract (FSOT) |
placed during revision endoscopic frontal sinusotomy. The middle turbinate had been previously resected and the remnant fragment was destabilized and scarred to the lateral nasal wall
be positioned within the anterior ethmoid cavity between the lateral nasal wall and vertical strut of the middle turbinate or middle turbinate remnant (Fig. 34.4). Proper positioning is best achieved using a 0 or 30 rigid endoscope and Blakesley forceps. This technique can also be applied to rolled Silastic sheeting; however, the stent needs to be fixed to the septum with nondissolvable through-and-through sutures to prevent extrusion.
Postoperative Stent Management
Expert opinion and experience indicates that regular debridement of the nasal cavity and stent itself is warranted. Regular nasal irrigations also play a key role in limiting the buildup of debris.
■Routine care of the stent, regardless of the material and placement technique, are necessary to maintain stent patency, minimize scarring, crusting, and adhesions, and improve long-term results.
■Use topical and/or oral steroids to reduce postoperative inflammation and scar formation around the stent.
■Routine empiric antibiotic coverage for the duration of the stenting is not recommended. Culture-directed antibiotics are used if purulent drainage occurs postoperatively.
■Stents aggregate biofilms given time.
■If purulent drainage persists despite appropriate medical therapy, consider removing the stent.
Even during the early days of frontal sinus stenting, Goodale and Harris routinely probed and cleaned the tantalum tubes with a curved suction [7, 8, 10].
Depending on surgeon preferences, nasal irrigation usually begins within the first few postoperative days and is maintained for at least the duration of stenting. Largevolume irrigations (50 ml twice daily) with a Toomey syringe are employed to manually dislodge crusts. Routine postoperative endoscopic removal of blood clots, crusting, dried secretions, and granulation tissue from within the nasal cavity and within the stent itself, should be performed in the office on a scheduled basis.
The use of topical and/or oral steroids is recommended to reduce postoperative inflammation and scar formation around the stent. Weber and Rains both employed topical inhaled nasal steroids as part of their postoperative medical regimen. [20, 23] However, in the absence of other sinonasal indications for oral or topical steroid use (i.e., sinonasal polyposis, mucosal edema, allergic fungal sinusitis), the presence of a healthy stent alone is not an absolute indication for utilization of these medications.
Routine empiric antibiotic coverage for the duration of the stenting is not recommended. However, appropriate culture-directed antibiotic therapy to control purulent drainage identified during routine endoscopy or as part of an episode of acute frontal sinusitis is warranted. If purulent drainage persists despite appropriate medical therapy, the stent may act as a foreign body or reservoir for biofilms, and consideration should be given to removing it. In a pilot study by Perloff and Palmer, six frontal sinus stents removed 1–4 weeks after functional endoscopic sinus surgery (FESS) and examined by scanning electron microscopy demonstrated evidence of bacterial biofilms [19]. In five of six of their patients, various staphylococcal species were cultured from the sinuses at the time of FESS.
FSOT stenting is an extremely safe procedure when performed by experienced endoscopic surgeons. No cases
Stenting in Revision Sinus Surgery
of orbital penetration, CSF leaks, or skull-base violations have been reported in the literature to date. In rare instances, some stents have required removal under general anesthesia due to significant scarring that obscures visualization and instrumentation in an office setting [3, 5, 6, 24]. However, one case of toxic shock syndrome due to frontal sinus stenting, despite 7 days of postoperative antibiotic prophylaxis, has been reported [4].
Conclusion
Frontal sinus stenting may play a role in specific cases of revision frontal sinus surgery. In cases of extensive mucosal trauma, bone exposure, or destabilized middle turbinate, stenting may improve long-term FSOT patency and enhance mucosalization. Regardless of the specific stent chosen, those made of soft Silicone are preferred to more rigid materials. Stenting may be employed during revision external or endoscopic frontal sinus surgery and is at the discretion of the surgeon. The appropriate duration of stenting has not been defined in the literature and should be determined on a case-by-case basis based upon patient characteristics, findings on CT imaging, and intraoperative observations. Appropriate postoperative stent management is crucial in maintaining long-term stent patency and consists of routine irrigation, endoscopic debridement, and appropriate antibiotic therapy for evidence of purulence. If granulation tissue and purulence persist despite adequate medical therapy, then consideration should be given to stent removal.
Acknowledgments
The authors would like to thank Martin J. Citardi, MD and Pete S. Batra, MD for providing clinical images for the figures.
References
1.Amble FR, Kern EB, Neel B, et al. (1996) Nasofrontal duct reconstruction with silicone rubber sheeting for inflammatory frontal sinus disease: analysis of 164 cases. Laryngoscope 106:809–815
2.Barton RT (1972) Dacron prosthesis in frontal sinus surgery. Laryngoscope 82:1795–1802
3.Benoit CM, Duncavage JA (2001) Combined external and endoscopic frontal sinusotomy with stent placement: a retrospective review. Laryngoscope 111:1246–1249
4.Chadwell JS, Gustafson LM, Tami TA (2001) Toxic shock syndrome associated with frontal sinus stents. Otolaryngol Head Neck Surg 124:573–574
307
5.Dubin MG, Kuhn FA (2006) Preservation of natural frontal sinus outflow in the management of frontal sinus osteomas. Otolaryngol Head Neck Surg 134:18–24
6.Freeman SB, Blom ED (2000) Frontal sinus stents. Laryngoscope 110:1179–1182
7.Goodale RL (1945) The use of tantalum in radical frontal sinus surgery. Ann Otol Rhinol Laryngol 45:757–762
8.Goodale RL (1954) Ten years’ experience in the use of tantalum in frontal sinus surgery. Laryngoscope 64:65–72
9.Har El G, Lucente FE (1995) Endoscopic intranasal frontal sinusotomy. Laryngoscope 105:440–443
10.Harris HE (1948) The use of tantalum tubes in frontal sinus surgery. Cleve Clin Q 15:129–133
11.Hosemann W, Kuhnel TH, Held P, et al. (1997) Endonasal frontal sinusotomy in surgical management of chronic sinusitis: A critical evaluation. Am J Rhinol 11:1–19
12.Hoyt WH (1993) Endoscopic stenting of nasofrontal communication in frontal sinus disease. Ear Nose Throat J 72:596–597
13.Ingals EE (1905) New operation and instruments for draining the frontal sinus. Trans Am Laryngol Rhinol Otol Soc 11:183–189
14.Jacobs JB (1997) 100 years of frontal sinus surgery. Laryngoscope 107:1–36
15.Lynch RC (1921) The technique of radical frontal sinus surgery operation which has given me the best results. Laryngoscope 31:1–5
16.Mirza S, Johnson AP (2000) A simple and effective frontal sinus stent. J Laryngol Otol 114:955–956
17.Neel HB, Whicker JH, Lake CF (1976) Thin rubber sheeting in frontal sinus surgery: animal and clinical studies. Laryngoscope 86:524–536
18.Neel HB, McDonald TJ, Facer GW (1987) Modified Lynch procedure for chronic frontal sinus diseases: rationale, technique, and long-term results. Laryngoscope 97:1274–1279
19.Perloff JR, Palmer JN (2004) Evidence of bacterial biofilms on frontal recess stents in patients with chronic rhinosinusitis Am J Rhinol 18:377–380
20.Rains BM (2001) Frontal sinus stenting. Otolaryngol Clin North Am 34:101–110
21.Schaefer SD, Close LG (1990) Endoscopic management of frontal sinus disease. Laryngoscope 100:155–160
22.Scharfe ED (1953) The use of tantalum in otolaryngology. Arch Otolaryngol 58:133–140
23.Weber R, Mai R, Hosemann W, et al. (2000) The success of 6-month stenting in endonasal frontal sinus surgery. Ear Nose Throat J 79:930–932, 934, 937–938, 940–941
24.Wish B, Dargi Z, Collins W, et al. (2006) Long-term effect of stenting after endoscopic modified Lothrop procedure. Am J Rhinol 20:595–599
25.Yamasoba T, Kikuchi S, Higo R (1994) Transient positioning of a silicone T tube in frontal sinus surgery. Otolaryngol Head Neck Surg 111:776–780
Chapter 35 |
35 |
Use of Intravenous Antibiotics |
|
in Sinus Surgery Failures |
Seth M. Brown, Abtin Tabaee, and Vijay K. Anand
|
Core Messages |
Contents |
|
||
|
|
|
■The management of patients with refractory chronic rhinosinusitis despite adequate surgical and medical treatment remains challenging.
■Intravenous antibiotics may play a role in patients who are considered “surgical failures,” particularly in those who demonstrate hyperostosis on imaging.
■Intravenous antibiotics are also indicated in intraorbital and intracranial complications of sinusitis in both adult and pediatric patients.
■Methicillin-resistant Staphylococcus aureus is increasing in prevalence as a pathogen in communityacquired sinusitis.
■The trend in intravenous antibiotics is toward home therapy with peripherally inserted central catheters.
■Preliminary studies support the cost-effectiveness and safety of home intravenous antibiotic treatment.
Introduction
Despite advances in the medical and surgical management of chronic rhinosinusitis (CRS), a subset of patients continues to have refractory symptoms despite “maximal therapy.” This group of patients represents a significant management challenge in the field of rhinology. Multiple treatment modalities are described including long-term broad-spectrum oral antibiotics, topical antibiotics, and anti-inflammatory medications [1]. For the patient with recalcitrant disease, this involves additional cost and potential treatment-related complications, with limited overall efficacy. The goal of this chapter is to describe the indications, safety, and outcomes of intravenous antibiotics in this cohort of patients. Given the significant incidence in the general population, negative impact on quality of life, and health-care expenditure associated
Introduction . . . . . . . . . . . . . . . . . |
309 |
Indications for Intravenous Antibiotics . . . . . |
. 309 |
Effectiveness of Intravenous Antibiotics . . . . . |
. 310 |
Pediatric Intravenous Antibiotic Treatment . . . . |
312 |
Method of Delivery of Home-Infused Intravenous |
|
Antibiotics . . . . . . . . . . . . . . . . . . |
313 |
Complications of Intravenous Antibiotics . . . . |
. 313 |
Benefits of Intravenous Antibiotics . . . . . . . |
. 314 |
Conclusion . . . . . . . . . . . . . . . . . |
. 314 |
with CRS, a critical examination of any treatment modality is required prior to widespread adoption.
Indications for Intravenous Antibiotics
1.Refractory rhinossinusitis.
2.Hyperostotic rhinossinusitis.
3.Extranasal complications from rhinossinusitis.
4.Resistant pathogens to oral antibiotics.
5.Patient intolerance to oral antibiotics.
6.Surgical alternative.
7.Failure of surgical therapy.
The indications for intravenous antibiotics in sinonasal disorders are currently in evolution. Traditional indications have included rhinossinusitis with intraorbital or intracranial complications and microbial resistance to oral antibiotics on sinonasal cultures, including methicil- lin-resistant Staphylococcus aureus (MRSA). Additional indications may include patients with medical comorbidities who are not medically fit for surgery and patients who have failed traditional medical and surgical therapy [8]. The use of antibiotics in the latter group remains controversial. The refractory nature of CRS in this cohort is
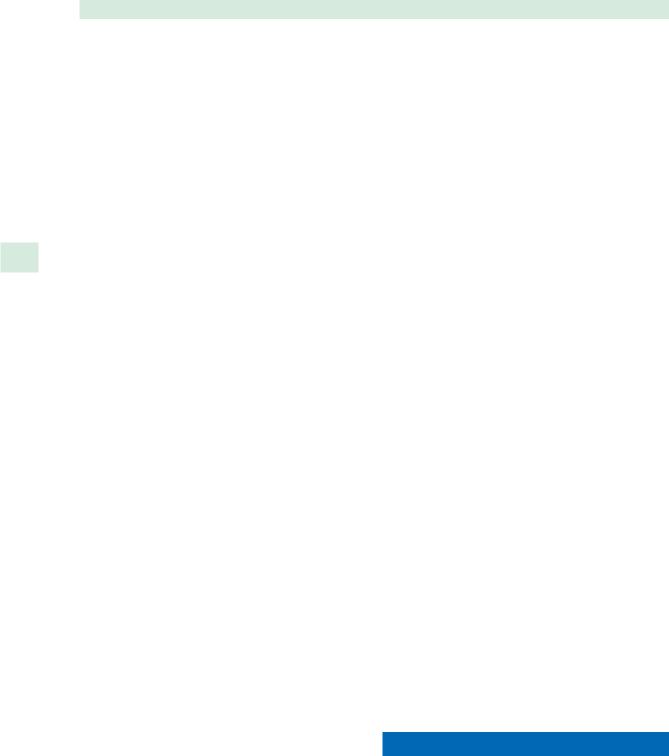
310
evidenced by the typical history of multiple courses of oral antibiotics and surgical procedures with persistent symptoms [14, 18]. The potential benefits of intravenous therapy in this group of patients have been described in a limited number of studies [8, 13, 18]. Intravenous antibiotics may be initiated independently or as an adjunct to surgical treatment with placement of a peripherally inserted central catheter (PICC) line in the early postoperative period [8]. In this model, treatment is initiated based on the intraoperative cultures and may include combinations of vancomycin, ceftriaxone, ceftazidime, and ciprofloxacin [8].
■ Obtaining and reviewing a computed tomography 35 (CT) scan is essential in those patients that have failed
surgery.
■The presence of hyperostosis on CT scan may suggest the need for intravenous antibiotics.
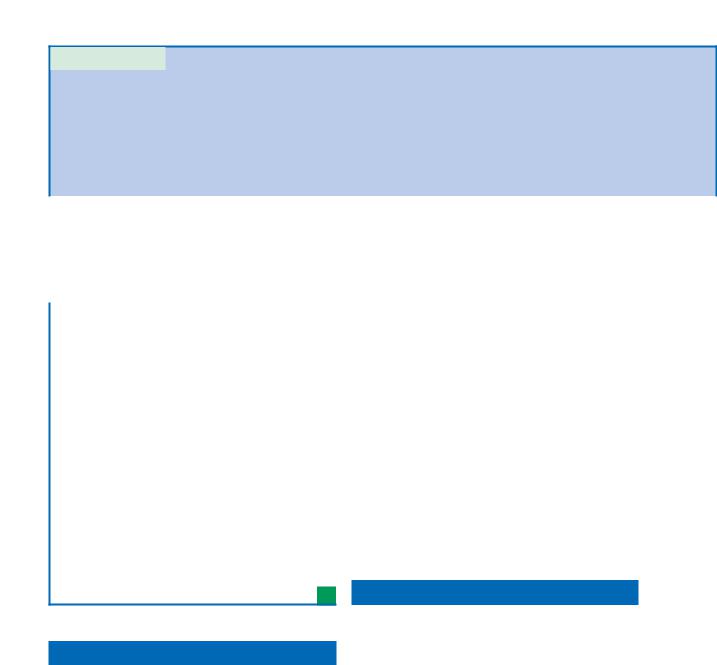
For patients with persistent symptoms following surgery, a critical evaluation of a CT scan is necessary to identify mucosal thickening, persistent obstruction of outflow tracts, and diffuse bony changes, termed hyperostosis (Fig. 35.1). The latter finding is felt to result from periosteal reactions, chronic inflammatory cell infiltrates, bony resorption, and osteoneogenesis [12]. Although bacteria have not been identified within the bone to date, these changes have been found to correlate with persistent sinusitis symptoms resistant to conventional medical management, including oral antibiotics [12].
The CT scan in patients with refractory CRS allows the surgeon to determine whether a patient would benefit from additional surgery in cases of polypoid disease or sinonasal obstruction. The role of surgery in hyperostosis alone is unclear. In cases of hyperostosis, often the mucosal disease is limited, although patients may report localized pain to the areas of bony abnormalities. Hyperostosis has also been found to correlate strongly with the presence of massive polyposis (Fig. 35.2) [12]. The presence of hyperostosis may also be a marker of refractory disease. In a retrospective review of a single surgical series, Kacker et al. identified hyperostosis in 65% of patients undergoing revision surgery, compared to 20% in patients undergoing primary surgery [12]. In another multi-institutional study of patients treated with intravenous home-based antibiotic administration for rhinosinusitis, 44 out of 52 had undergone previous sinus surgery [2]. Looking at symptom analysis, significant improvement was seen in these patients when previous treatment with oral antibiotics and/or surgery had failed [2].
■Intravenous antibiotics may represent a safe and effective treatment alternative for patients with MRSA sinusitis.
Seth M. Brown, Abtin Tabaee, and Vijay K. Anand
The increasing incidence of microbial pathogens resistant to currently available oral antibiotics, typically MRSA, represents a major public health concern. The majority of patients with MRSA sinusitis have a known risk factor including a history of hospitalization and multiple treatments including antibiotics and surgeries. The incidence of MRSA in patients with refractory CRS is currently estimated to be 2–15% and is likely increasing [10, 11, 14]. In a study of all sinonasal cultures taken in the senior author’s tertiary referral rhinology practice, 9.2% of patients were found to have MRSA [14]. The treatment options for medical therapy in this cohort are limited. In a subsequent study of intravenous antibiotics for treatment of MRSA sinusitis in this group, negative cultures were achieved in five of six patients at last follow up after a 6- to 8-week treatment course. The single patient with persistent cultures was noted to have improvement in clinical and endoscopic disease, suggesting the possibility of MRSA colonization without pathogenicity [18]. These results are further supported when looking at 20-item Sinonasal Outcome Test (SNOT-20) scores. In a study by Tabaee et al., SNOT-20 scores improved from a pretreatment median of 62, to 42 after a 6- to 8-week course of intravenous antibiotics for MRSA [18].
The role of intravenous therapy in the treatment of MRSA sinusitis is currently unclear. We advocate a treatment algorithm based on culture-directed sensitivities (Fig. 35.3). If sensitivity to an oral antibiotic is suggested, this is employed initially, often in combination with a second broad-spectrum antibiotic with anaerobic and Gramnegative coverage. Intravenous antibiotics are initiated if there is persistent clinical disease following a trial of oral therapy, or if the initial culture results show resistance to all oral alternatives. The role of intravenous antibiotics will probably continue to evolve as newer classes of oral antibiotics become available, such as linezolid. Other studies have promoted using mupirocin nasal irrigations for MRSA; however, recurrence rates appear high, with 12 of 24 patients experiencing at least 1 recurrence in 1 series [17]. Finally, despite the potential efficacy of culturedirected therapy for MRSA rhinossinusitis, long-term surveillance for this cohort is required. Repeat cultures should be performed in patients with recurrent disease even if the posttreatment cultures were negative.
Effectiveness of Intravenous Antibiotics
■Intravenous antibiotics appear to be effective for surgical failures or as an adjunct to surgery, but may be far less effective when used as an alternative to surgery.
The efficacy of intravenous antibiotics in conjunction with surgery has been suggested by two recent studies. In a
Use of Intravenous Antibiotics in Sinus Surgery Failures |
311 |
Fig. 35.1 Hyperostosis seen on computed tomography (CT). a Axial. b Coronal. c Sagittal
multi-institutional study of 52 patients with CT evidence of CRS with hyperostosis, Anand et al. reported significant clinical, endoscopic, and radiographic improvement following a course of culture-directed intravenous antibiotics [2]. In this study, the Rhinosinusitis Disability Index was used as one form of assessment and showed an improvement after treatment in average score (from 67.7
to 19.3), physical domain (from 25.4 to 7.7), functional domain (from 23.0 to 6.6), and emotional domain (from 19.3 to 5.0) [2]. In a separate study, Gross et al. showed a partial or complete response in 11 of 14 patients following surgery and culture-directed intravenous antibiotics [8]. Interestingly, the use of culture-directed intravenous antibiotics as an alternative to surgery is not supported
312 |
Seth M. Brown, Abtin Tabaee, and Vijay K. Anand |
35
Fig. 35.2 Hyperostosis seen in patient with massive polyposis. a Axial CT. b Coronal CT
Fig. 35.3 Demonstration of endoscopic-directed culture
by the available literature. In a study of patients receiving a course of intravenous antibiotic therapy without surgery, Fowler et al. found an 89% relapse rate at a mean of 11.5 weeks after treatment [5].
In summary, the available literature, although limited, may support the use of culture-directed intravenous antibiotics in patients with refractory disease following maximal medical therapy and surgery. Treatment duration has been arbitrary in the literature, but for cases of surgical failure with hyperostosis, a 6- to 8-week course may be
appropriate, similar to the treatment for osteomyelitis of the extremities [2]. Additional clinical and basic science investigation is required prior to definitive treatment guidelines.
Pediatric Intravenous Antibiotic Treatment
■The limited studies investigating intravenous antibiotics for pediatric rhinossinusitis suggest overall effectiveness, but a significant incidence of complications.
Don et al. reported an 89% success rate in pediatric patients with CRS aged 10 months to 15 years treated with 1–4 weeks of culture-directed intravenous antibiotics and select adenoidectomies [4]. Endoscopic sinus surgery was reserved for patients failing this treatment course. It is also important to note that the majority of patients in the study (67%) had continuation of therapy with oral antibiotics after completion of the intravenous therapy. The authors noted correlations between treatment failure and older patient age, and longer preexisting symptoms [4]. Although the authors did not report data on antibiotic or catheter complications, a separate report suggested higher complication and discontinuation rates for homebased intravenous therapy in children than in adults [7]. However, in the same study they concurred with the high success rates in children, reporting excellent outcomes even in pediatric patients with early discontinuation of treatment [7].
