
Revision Sinus Surgery
.pdf
292
reduce misdiagnosis, such as changes related to free fat grafts used in prior reconstruction.
Scar and other high signal tissues may not be easily discernable from recurrent disease. Changes on serial examinations, after an early postoperative baseline study, may be the only characteristic features of residual pathology [43]. MRI also enables differentiation of tumor from retained secretions.
Magnetic resonance angiography or CT angiography 33 (Fig. 33.2) [36] can be used to assess the structure of me- dium-to-large arteries in patients when erosion of the sphenoid and clivus has occurred. The relationship between the basilar arteries and ICAs to pathology is crucial in this area. Although conventional angiography is not routinely performed, it may verify the functional integrity of the circle of Willis, the extent of any carotid artery
compromise, and differentiate aneurysm from tumor.
Endocrine and Hypothalamic Considerations
Even with the best preoperative imaging, the relationship between tumor and intracranial structures can be difficult to demonstrate. It is extremely difficult to evaluate before surgery whether some tumors are extra-arachnoi- dal, extrapial-subarachnoidal, or partially intraparenchy- mal-subpial [16]. This weighs heavily on considerations to obtain complete resection. Parasellar pathologies such as craniopharyngiomas may often encase the pituitary
Aldo C. Stamm, João Flávio, and Richard J. Harvey
stalk or involve the hypothalamus. Resection of the pituitary stalk carries considerable morbidity with hormone replacement and reproductive dysfunction for adults, and has an even greater impact on growth and development for children. Hypothalamic injury may result in severe, crippling and life-threatening sequelae, such as adipsia, morbid obesity, sleep disturbances, and behavioral and cognitive disorders [56]. The decision to attempt complete tumor removal when resection or injury to these structures is inevitable should be discussed with the patient prior to surgery and not left to an intraoperative dilemma when such circumstances arise [17]. Careful preoperative assessment with an endocrinologist, within an MDT, for pituitary and hypothalamic dysfunction is essential in the operative planning for these patients [40, 48, 56].
Instrumentation
Endoscopic SBS is highly dependant on specialized instrumentation and its absence is an absolute contraindication to surgery. Dissection instruments and drills that are appropriately long enough to perform dissection beyond the sphenoid are essential. Similarly, high-quality endoscopes and video equipment are required. It is not appropriate to perform endoscopic SBS without camera equipment. An entire operative team needs to be visually informed of the state of surgery. A second surgeon, for bimanual dissection, and theater staff should be actively involved. The ability to maintain hemostasis and
Fig. 33.2 Computed tomography angiogram of a recurrent clival chordoma. Note the laterally displaced position of the internal carotid arteries (ICAs). (Reproduced with permission from Centro de ORL, Sao Paulo, Brazil)

Revision Endoscopic Skull-Base Surgery |
293 |
Fig. 33.3 Endoscopic skull-base surgery equipment. a 5.2-mm and 4-mm endoscopes. b Image-guidance systems. c Long bi-
a workable operative field during prolonged surgery is a demanding aspect of extended endoscopic surgery. Long endoscopic bipolar forceps and hemostatic materials such as Surgicel or Avatine are essential to this process (Fig. 33.3) [28].
Previous Radiotherapy
Precise knowledge of previous radiotherapy fields will have significant impact on reconstructive options. In irradiated tissue, multilayered free graft repair of skull-base defects has a high dehiscence rate in our experience and elsewhere [45]. Reconstruction of the irradiated skull base almost always demands vascularized tissue. Pedicled mucoperiosteal and mucoperichondrial flaps are used for reconstruction. Radiotherapy may also thicken the pia arachnoid tumor interface, obscuring dissection planes, and contribute to a greater risk of neural injury [43].
polar forceps. d Long protected drill shafts. (Reproduced with permission from Centro de ORL, Sao Paulo, Brazil)
Surgical Techniques
General Principles
■The medial orbital wall, orbital apex, and prechiasmal optic nerve axis is a key landmark in revision SBS.
■Dissection should always proceed from known to unknown.
Endoscopic dissection in a previously operated skull base can be challenging due to the loss of traditional surgical landmarks. The use of image guidance has gained increasingly popularity since early reports of its use [6]. Improving the accuracy of image guidance has an associated learning curve [55] and occasional use may prove frustrating to the unfamiliar. Fusion MRI and CT guidance may provide more significant information for the surgeon operating on the skull base with extensive bone loss and altered soft-tissue structures [34]. However, detailed

296 |
Aldo C. Stamm, João Flávio, and Richard J. Harvey |
33
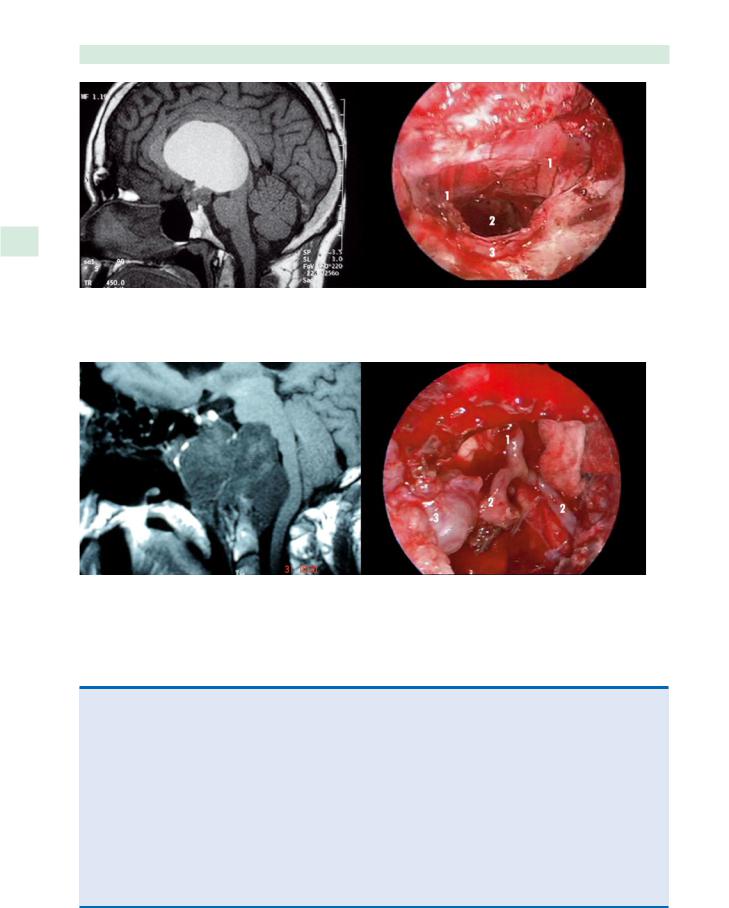
Fig. 33.5 Recurrent cystic craniopharyngioma. 1 Olfactory tract, 2 transplanum opening to cyst, 3 Sella. (Reproduced with permission from Centro de ORL, Sao Paulo, Brazil)
Fig. 33.6 Recurrent chordoma. 1 Basilar artery, 2 vertebral arteries, 3 tumor. (Reproduced with permission from Centro de ORL, Sao Paulo, Brazil)
Table 33.3 Graft materials used in reconstruction of skull-base defects
•Mucosal flaps
–Posterior rotation septal flap (based on the septal branch of the sphenopalatine artery (SPA); Fig. 33.7a)
–Contralateral transposition septal flap (based on ethmoidal arteries; Fig. 33.7b)
–Inferior turbinate flap (based on turbinate branch of the SPA; Fig. 33.7c)
–Nasal floor flap (based on branches of the SPA and Woodruff’s plexus; Fig. 33.7d)
•Free mucosal or mucoperioteal grafts
–Well-described series with closure of anterior skull-base defects with cerebrospinal fluid leaks but not with extensive dural resection [35]
•Tissue glues and substrates – BioGlue [13] Tisseel [27] DuraGen [9] and Duraseal
•Autologus fascia (fascia lata, temporalis fascia)
•Homologus fascia (Alloderm)
•Free fat grafts
•Free flaps [67] (usually requiring conversion to open skull-base surgery)
•Free bone or synthetic materials





Chapter 34
Stenting in Revision Sinus Surgery |
34 |
Seth J. Kanowitz, Joseph B. Jacobs, and Richard A. Lebowitz |
|
|
|
|
Contents |
|
|
|
Core Messages |
|
|
|
|
|
|
|
||
|
■ Long-term patency rates may be improved by post- |
Introduction . . . . . . . . . . . . . . . . . |
301 |
||
|
Stenting Materials and Design . . . . . . . . . |
. 301 |
|||
|
■ |
operative stenting of the frontal sinus outflow tract. |
Preoperative Assessment . . . . . . . . . . . . |
303 |
|
|
In cases of previous partial middle-turbinate resec- |
Indications for Stenting . . . . . . . . . . . . |
. 303 |
||
|
|
tion, stenting of the frontal sinus outflow tract al- |
Duration of Stenting . . . . . . . . . . . . . |
. 304 |
|
|
|
lows for stabilization of the remnant fragment dur- |
|||
|
■ |
ing revision frontal sinusotomy. |
Surgical Technique . . . . . . . . . . . . . . |
. 305 |
|
|
In cases of extended frontal sinus drillout proce- |
Postoperative Stent Management . . . . . . . . |
. 306 |
||
|
|
dures, stenting allows for improved mucosalization |
Conclusion . . . . . . . . . . . . . . . . . |
. 307 |
|
|
|
and aids in temporary inhibition of circumferential |
|||
|
|
Acknowledgments . . . . . . . . . . . . . |
. 307 |
||
|
|
stenosis. |
|||
■Soft (Silicone) sheets or stents, either prefabricated or designed in the operating room, are superior to rigid stents.
■No absolute length of stenting exists and a determi- ing endoscopic visualization, high-resolution triplanar
nation should be made on a case-by-case basis. |
computed tomographic (CT) imaging, through-cutting |
|||
■ Postoperative stent management includes routine |
frontal sinus instrumentation, and image-guided surgery |
|||
endoscopy with gentle debridement, culture-direct- |
have occurred since the original external approaches |
|||
ed antibiotic therapy, and nasal irrigations. Nasal |
were described, prevention of FSOT restenosis after revi- |
|||
steroid spray is reserved for select cases. |
sion sinus surgery remains a difficult challenge. |
|||
|
|
|
Intrinsic host factors such as sinonasal polyposis, |
|
|
|
|
||
|
|
|
osteoneogenesis, ciliary dyskinesis, immunodeficiency, |
|
|
|
|
vasculitis, and other autoimmune phenomena may pre- |
|
|
|
|
dispose the patient to a poor outcome regardless of the |
|
|
|
|
surgical technique. Extrinsic factors such as lateralization |
|
Introduction |
|
|||
|
of the middle turbinate/middle turbinate remnant, post- |
|||
|
|
|
||
|
|
|
||
Revision frontal sinus surgery is a demanding challenge |
operative sinus cavity infections, scarring, synechiae, and |
|||
that incorporates a keen understanding of three-dimen- |
incomplete primary sinus surgery may also compromise |
|||
sional anatomy, surgical precision, and vigorous post- |
postoperative healing and ultimately lead to FSOT ste- |
|||
operative medical management aimed at maximizing |
nosis. Historically, failure rates of nearly 30% have been |
|||
long-term surgical success. Restenosis of the frontal sinus |
reported in the literature – and because of this propensity |
|||
outflow tract (FSOT) is frustrating and can occur even |
for postoperative stenosis of the FSOT, stenting remains |
|||
under the best of circumstances. The concept of frontal |
an important component in the surgical and postopera- |
|||
sinus stenting to minimize postoperative stenosis, im- |
tive management of chronic frontal sinusitis during revi- |
|||
prove mucosalization, and allow for functional patency |
sion endoscopic surgery. |
|||
of the of the FSOT following frontal sinus surgery has |
|
|||
been reported in the literature for over a century. In the |
|
|||
initial description of the external frontoethmoidectomy |
|
|||
Stenting Materials and Design |
||||
that now bears his name, Lynch described postoperative |
||||
|
||||
stenting of the nasofrontal communication. Although |
From a historical perspective, the initial descrip- |
|||
many technological advances in sinus surgery includ- |
tions of frontal sinus stenting usually involved external |
|||
