
- •12.1 The Primary Structure of Nucleic Acids
- •12.2 The abZs of dna Secondary Structure
- •Intercalating Agents Distort the Double Helix
- •12.3 Denaturation and Renaturation of dna
- •12.4 Supercoils and Cruciforms: Tertiary Structure in dna
- •12.5 Chromosome Structure
- •12.6 Chemical Synthesis of Nucleic Acids
- •12.7 Secondary and Tertiary Structure of rna
- •Isopycnic Centrifugation and Buoyant Density of dna
Isopycnic Centrifugation and Buoyant Density of dna
Density gradient ultracentrifugation is a variant of the basic technique of ultracentrifugation (discussed in the Appendix to Chapter 5). Density gradient centrifugation can be used to isolate DNA. The densities of DNAs are about the same as concentrated solutions of cesium chloride, CsCl (1.6 to 1.8 g/mL). Centrifugation of CsCl solutions at very high rotational speeds, where the centrifugal force becomes 105 times stronger than the force of gravity, causes the formation of a density gradient within the solution. This gradient is the result of a balance that is established between the sedimentation of the salt ions toward the bottom of the tube and their diffusion upward toward regions of lower concentration. If DNA is present in the centrifuged CsCl solution, it moves to a position of equilibrium in the gradient equivalent to its buoyant density (Figure A12.1). For this reason, this technique is also called isopycnic centrifugation.
F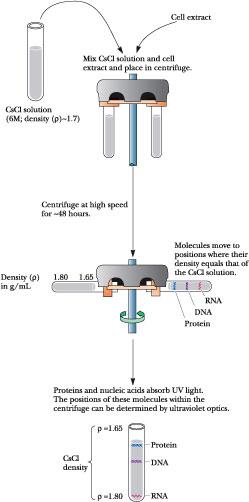 igure
A12.1
•
Density gradient centrifugation is a common method of separating
macromolecules, particularly nucleic acids, in solution. A cell
extract is mixed with a solution of CsCl to a final density of about
1.7 g/cm 3
and
centrifuged at high speed (40,000 rpm, giving relative centrifugal
forces of about 200,000 g).
The biological macromolecules in the extract will move to equilibrium
positions in the CsCl gradient that reflect their buoyant densities.
igure
A12.1
•
Density gradient centrifugation is a common method of separating
macromolecules, particularly nucleic acids, in solution. A cell
extract is mixed with a solution of CsCl to a final density of about
1.7 g/cm 3
and
centrifuged at high speed (40,000 rpm, giving relative centrifugal
forces of about 200,000 g).
The biological macromolecules in the extract will move to equilibrium
positions in the CsCl gradient that reflect their buoyant densities.
Cesium chloride centrifugation is an excellent means of removing RNA and proteins in the purification of DNA. The density of DNA is typically slightly greater than 1.7 g/cm3, while the density of RNA is more than 1.8 g/cm3. Proteins have densities less than 1.3 g/cm3. In CsCl solutions of appropriate density, the DNA bands near the center of the tube, RNA pellets to the bottom, and the proteins float near the top. Single-stranded DNA is denser than double-helical DNA. The irregular structure of randomly coiled ssDNA allows the atoms to pack together through van der Waals interactions. These interactions compact the molecule into a smaller volume than that occupied by a hydrogen-bonded double helix. The net movement of solute particles in an ultracentrifuge is the result of two processes: diffusion (from regions of higher concentration to regions of lower concentration) and sedimentation due to centrifugal force (in the direction away from the axis of rotation). In general, diffusion rates for molecules are inversely proportional to their molecular weight—larger molecules diffuse more slowly than smaller ones. On the other hand, sedimentation rates increase with increasing molecular weight. A macromolecular species that has reached its position of equilibrium in isopycnic centrifugation has formed a concentrated band of material. Essentially three effects are influencing the movement of the molecules in creating this concentration zone: (1) diffusion away to regions of lower concentration; (2) sedimentation of molecules situated at positions of slightly lower solution density in the density gradient; and (3) flotation (buoyancy or “reverse sedimentation”) of molecules that have reached positions of slightly greater solution density in the gradient. The consequence of the physics of these effects is that, at equilibrium, the width of the concentration band established by the macromolecular species is inversely proportional to the square root of its molecular weight. That is, a population of large molecules will form a concentration band that is narrower than the band formed by a population of small molecules. For example, the band width formed by dsDNA will be less than the band width formed by the same DNA when dissociated into ssDNA.
