
3 курс / Фармакология / Essential_Psychopharmacology_2nd_edition
.pdf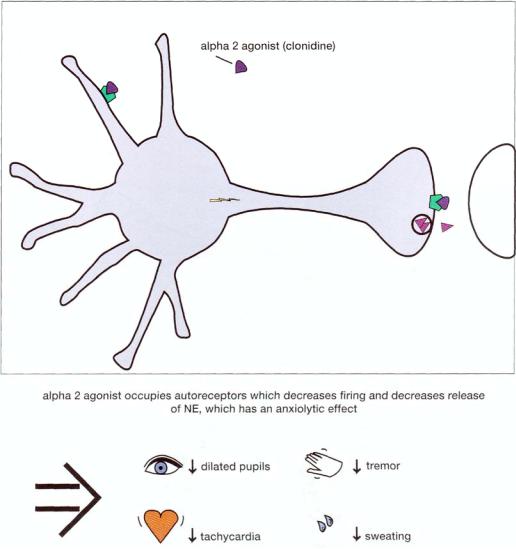
Anxiolytics and Sedative-Hypnotics |
309 |
FIGURE 8—13. If an alpha 2 agonist such as clonidine, is administered, it will have much the same action as norepinephrine (NE) itself both at somatodendritic alpha 2 autoreceptors and at terminal alpha 2 autoreceptors. This action is that of reducing both neuronal impulse in NE neurons and release of NE from noradrenergic axon terminals. Thus, alpha 2 agonists will decrease the symptoms
associated with anxiety, especially the autonomic symptoms of dilated pupils, tachycardia, tremor, and sweating.
voked during detoxification of patients from alcohol, barbiturates, heroin, or benzodiazepines (see Chapter 13).
Overactivity of noradrenergic neurons creates too much postsynaptic norepinephrine at noradrenergic receptors, particularly beta receptors (Fig. 8—14). As is consistent with the hypothesis of a state of norepinephrine excess in anxiety, it is possible to reduce symptoms of anxiety in some cases by blocking beta receptors with beta

FIGURE 8—14. The overactivity of norepinephrine (NE) neurons and excess release of NE from nerve terminals that occurs in anxiety also causes events to occur at postsynaptic NE receptors. In this case, excess NE activity causes an excess of NE occupying postsynaptic beta adrenergic receptors. This in turn causes an excess in postsynaptic signaling via second and subsequent messenger systems. These excess signals via postsynaptic beta adrenergic receptors mediate the autonomic symptoms associated with anxiety, including tachycardia, dilated pupils, tremor, and sweating.
310

Anxiolytics and Sedative-Hypnotics |
311 |
FIGURE 8-15. If excessive activity of norepinephrine (NE) at postsynaptic beta adrenergic receptors, shown in Figure 8 — 14, can be blocked, so can the symptoms of anxiety that are mediated by the beta receptors. Shown here is a beta adrenergic blocker, or antagonist, which is preventing the excess activity of NE neurons and excess NE release to cause a corresponding excess stimulation of postsynaptic beta adrenergic receptors. This antagonist action at beta receptors can decrease the autonomic symptoms associated with anxiety, such as dilated pupils, tremor, tachycardia, and sweating.
blocking drugs (Fig. 8-15). This may be especially useful in cases of social phobia, as will be discussed in Chapter 9.
GABAergic Neurons and the Benzodiazepine Anxiolytics
Understanding the actions of benzodiazepines requires background knowledge of the pharmacology of GABA-ergic neurotransmission. The neurotransmitter for GABA-
312 Essential Psychopharmacology
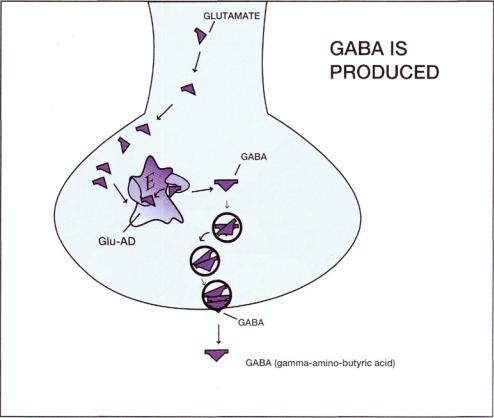
FIGURE 8—16. Gamma-aminobutyric acid (GABA) is produced by synthesis from the precursor amino acid glutamate by the enzyme glutamic acid decarboxylase (Glu-AD).
ergic neurons is GABA, which is synthesized from the amino acid precursor glutamate via the enzyme glutamic acid decarboxylase (Glu-AD) (Fig. 8—16). Glutamate (glutamic acid) is derived from intraneuronal stores of amino acids. It is a nonessential amino acid and is the most abundant free amino acid in the central nervous system (CNS). Glutamate also participates in multiple metabolic functions and can be synthesized from numerous precursors (see discussion of glutamate neurons in Chapter 10). The GABA neuron has a presynaptic transporter (reuptake pump) (Fig. 8—17) similar to those of norepinephrine, dopamine, and serotonin|and discussed in Chapter 5. This transporter terminates the action of synaptic GABA by removing it from the synaptic cleft for restorage or for destruction by the enzyme GABA transaminase (GABA-T) (Fig. 8-17).
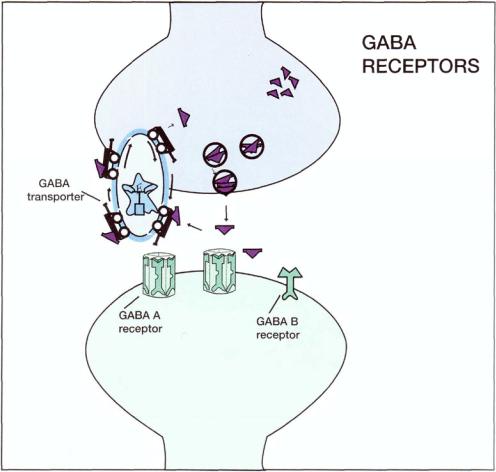
Receptors for GABA also regulate GABA-ergic neurotransmission. There are two known subtypes of GABA receptors, known as GABA A and GABA B (Fig. 8—18). The GABA A receptors are the ones that are gatekeepers for a chloride channel (Figs. 8—19 and 8 — 20). They are allosterically modulated by a potpourri of nearby receptors. This includes the well-known benzodiazepine receptor (Fig. 8 — 19). The concept of allosteric modulation of one receptor by another has already been introduced in Chapter 3.

Anxiolytics and Sedative-Hypnotics |
313 |
FIGURE 8 — 17. The action of gamma-aminobutyric acid (GABA) is terminated either by enzymatic destruction by GABA transaminase (GABA T) or by removal from the synaptic cleft by the GABA transporter. Following transport back into the presynaptic neuron, GABA can be re-stored in synaptic vesicles for reuse during a subsequent neurotransmission, as already pointed out for the norepinephrine, dopamine, and serotonin neurons.
The fundamental neurobiological importance of the GABA A receptor is underscored by observations that even more receptor sites exist at or near this complex (Fig. 8 — 20). This includes receptor sites for nonbenzodiazepine sedativehypnotics such as zolpidem and zaleplon, for the convulsant drug picrotoxin, for the anticon-vulsant barbiturates, and perhaps even for alcohol. This receptor complex is hypo-thetically responsible in part for mediating such wide-ranging CNS activities as seizures, anticonvulsant drug effects, and the behavioral effects of alcohol, as well as the known anxiolytic, sedative-hypnotic, and muscle relaxant effects of the benzodiazepines.
A second GABA receptor subtype, the GABA-B receptor (Fig. 8 — 12) is not allosterically modulated by benzodiazepines but binds selectively to the muscle relaxant baclofen. Its physiological role is not well known yet, but does not appear to be closely linked to anxiety disorders or to anxiolytics.
Benzodiazepine receptors. There are multiple molecular forms of benzodiazepine receptors, and there is continuing debate about how differences in the amino acid com-
314 Essential Psychopharmacology
FIGURE 8-18. Various receptors for gamma-aminobutyric acid (GABA) are shown here at the GABA synapse. On the presynaptic side is the GABA transporter, the receptor linked to an active transport pump that removes GABA from the synaptic cleft, thus terminating its actions there. On the postsynaptic side, two subtypes of GABA receptors are shown. The first is the GABA A receptor, which is a member of the superfamily of ligand-gated ion channel receptors. The second is the GABA B receptor.
position of benzodiazepine receptors may lead to pharmacological differences in ligand binding and in functional activity. There may be at least five benzodiazepine receptor subtypes, including three with distinct pharmacologic profiles. For example, benzodiazepine 1 (sometimes called omega 1) receptors are preferentially located in the cerebellum and contain recognition sites with high affinities both for benzodiazepines and for agents with different chemical structures. Anxiolytic action as well as sedative-hypnotic actions seem to be mediated mostly through the benzodiazepine 1 receptor subtype. Benzodiazepine 2 (omega 2) receptors, on the other hand, are located predominantly in the spinal cord and striatum. These receptors may be involved in mediating the muscle relaxant actions of benzodiazepines. Finally, the

Anxiolytics and Sedative-Hypnotics |
315 |
FIGURE 8-19. The GABA A receptor is shown here. It acts as a gatekeeper for a chloride channel. It also has a key allosteric modulatory site nearby, known as the benzodiazepine (BZ) binding
site.
benzodiazepine 3 receptor, also known as the peripheral (i.e., outside the CNS) type, is abundant in the kidney. Its role in anxiolytic actions remains unclear.
Actions at benzodiazepine receptors are thought to underlie virtually all the pharmacological actions of the benzodiazepines, those that are desirable as well as those that are undesirable. This includes the desirable therapeutic actions of benzodiazepines as anxiolytics and sedative-hypnotics, as well as anticonvulsants and muscle relaxants. It also includes their undesirable side effects as amnestic agents and as agents that cause adaptations at the benzodiazepine receptor with chronic administration, which are thought to underlie the production of dependence and withdrawal from these agents (see Chapter 13).
Benzodiazepines are listed in the Table 8 — 2. The fact that they act at a naturally occurring receptor in brain has led to speculation that the brain may make its own benzodiazepine, or "endogenous Valium." Identification of a naturally occurring ligand for the benzodiazepine receptor, however, is yet incomplete. Modulation of the GABA—benzodiazepine receptor complex is therefore not only thought to underlie the pharmacological actions of antianxiety drugs but is also theorized to serve as the vehicle for mediating the emotion of anxiety itself. It has been speculated, for example, that reduced actions of GABA and the postulated endogenous benzodiaze-

316 Essential Psychopharmacology
FIGURE 8 — 20. Multiple modulatory sites near the GABA A receptor are shown here. These include not only the benzodiazepine (BZ) receptor already shown in Figure 8 — 19, but also sites for the convulsant drug picrotoxin, for the anticonvulsant barbiturate, and possibly for alcohol as well. These nearby receptors suggest how GABA may be involved in modulating such diverse physiological effects as anxiety, seizures, and even the effects of alcohol.
pines at this receptor complex may be associated with the emotion of anxiety, whether the emotion is normal or pathological.
Positive allosteric interactions between GABA A receptors and benzodiazepine receptors. In technical language, the benzodiazepines are positive allosteric modulators of fast inhibitory neurotransmission by GABA at GABA A receptors. The inhibitory neurotransmitter GABA is the gatekeeper neurotransmitter that interacts selectively with its GABA A receptor, the primary receptor site of this GABA—benzodiazepine chloride channel receptor complex shown (Fig. 8 — 19). Thus, this complex is an example of the superfamily of receptors already discussed in Chapter 3 (see Figs. 3 — 3 through 3 — 14) and known as ligand-gated receptor complexes. In the case of the GABA—benzodiazepine receptor complex, this particular superfamily complex acts to control a chloride ion channel mediating fast neurotransmission (see discussion in Chapter 1 and Fig. 1 — 6).

Anxiolytics and Sedative-Hypnotics |
317 |
Table 8 — 2. Some of the major benzodiazepines
Alprazolam (Xanax)
Clonazepam (Klonopin)
Diazepam (Valium)
Chlordiazepoxide (Librium)
Lorazepam (Ativan)
Oxazepam (Serax) Prazepam
(Centrex) Clorazepate
(Tranxene) Triazolam
(Halcion) Temazepam
(Restoril) Flurazepam
(Dalmane) Midazolam
(Versed) Quazepam (Doral)
Flumazenil (Romazicon)
Mitrazepam Lormetazolam
Loprazolam Clobazam
Flunitrazepam Brotizolam
The GABA A receptors are arranged as helical columns around a chloride channel, which itself is a column of columns, as mentioned in Chapter 3 and shown in Figures 3—6 through 3 — 12 as well as in Figures 8 — 18 through 8—20. Following occupancy of the GABA A receptor site by GABA molecules, the GABA A receptor columns in turn interact with the chloride channel to open it a bit (Fig. 8-21). The resulting increased chloride conductance into the neuron occurs quickly (fast neurotransmission; see Chapter 1 and Fig. 1—6) and is inhibitory to the finding of that neuron.
Near the receptor site for GABA is not only the chloride channel but also another neurotransmitter receptor, namely the benzodiazepine receptor binding site (Fig. 8—19), as already mentioned. Benzodiazepine receptor binding sites also affect the conductance of chloride through the chloride channel. However, the benzodiazepine receptor binding site does not do this by directly modulating the chloride channel but by allosterically modulating the GABA A receptor binding site, which in turn modulates the chloride channel. Thus, when a benzodiazepine binds to its own benzodiazepine receptor site, a neighbor of the GABA A receptor binding site, nothing happens if GABA is not also binding to its own GABA A receptor site (Fig. 8 — 22). On the other hand, when GABA is binding to its GABA A receptor site, the simultaneous binding of benzodiazepine to its benzodiazepine binding site (allosteric, i.e., "other site") causes a large amplification in GABA's ability to increase the conductance of chloride through the channel (Fig. 8 — 23).
Why is this necessary? It turns out that GABA working by itself can increase chloride conductance through the chloride channel only to a certain extent (Fig. 8—21). Benzodiazepines cannot increase chloride conductance at all when working by themselves (Fig. 8 — 22). However, allosteric modulation is the mechanism to maximize the chloride conductance beyond that which GABA alone, as in Figure

FIGURE 8 — 21. GABA is the ligand that acts at the GABA A receptor site to participate in opening the molecular gate for an inhibitory chloride channel. Thus, when GABA alone binds to the GABA A receptor, it opens the chloride channel so that more chloride can now enter the cell and cause inhibitory neurotransmission. Also shown is a benzodiazepine (BZ) ligand, which is not binding to the benzodiazepine receptor in this example (but see Figs. 8 — 22 and 8 — 23).
FIGURE 8 — 22. Shown here is a benzodiazepine (BZ) ligand binding to the benzodiazepine receptor on the GABA A receptor complex. Note that GABA itself is not binding to the GABA A receptor site in this example. Note also that the chloride channel is not opening. This example is thus in contrast to the previous example (Fig. 8 — 21), in which GABA alone was binding to the GABA A receptor. In the current example, where BZ ligand alone binds to the GABA A receptor, essentially no more chloride can enter the cell to cause inhibitory neurotransmission. However, see the contrast in Figure 8 — 23, in which both GABA and benzodiazepine bind to the GABA A receptor complex.
318
