
Обучение чтению литературы на английском языке по специальности «Аэродинамика». В 2 ч. Ч. 1 (96
.pdf
Copyright ОАО «ЦКБ «БИБКОМ» & ООО «Aгентство Kнига-Cервис»
УДК 802.0 ББК 81.2 Англ&923
А602
Рецензент З.А. Заболотская
Алявдина Н.Г., Шашмурина А.В.
А602 Обучение чтению литературы на английском языке по спе& циальности «Аэродинамика»: Учеб.&метод. пособие: В 2 ч. – Ч. 1. – М.: Изд&во МГТУ им. Н.Э. Баумана, 2007. – 36 с.: ил.
Пособие содержит оригинальные тексты из английских и американских научно-технических изданий, лексико-грамматические упражнения, способствующие развитию и закреплению навыков перевода литературы по специальности.
Для студентов 3&го курса факультета «Специальное машиностроение», обучающихся по специальности «Аэродинамика».
УДК 802.0 ББК 81.2 Англ&923
МГТУ им. Н.Э. Баумана, 2007
ПРЕДИСЛОВИЕ
В пособие включены оригинальные тексты из английской и аме& риканской научно&технической литературы; словари, содержащие активную лексику, главным образом терминологию; лексико&грам& матические упражнения, способствующие развитию и закреплению навыков понимания, осмысления, перевода и аннотирования литера& туры на английском языке по изучаемой специальности, а также на& выков устной речи, связанной с соответствующей тематикой.
Материал, представленный в пособии, может использоваться студентами как во время аудиторных занятий (под руководством преподавателя), так и в процессе самостоятельной работы.
Пособие предназначено для студентов старших курсов факультета «Специальное машиностроение».
|
UNIT I |
|
New Words and Word Combinations |
lead n |
свинец |
occur v |
иметь место, встречаться |
solid n |
твердое тело |
solid а |
твердый |
investigate v |
исследовать, изучать |
investigation n |
исследование |
to refer to |
упоминать ч.&л., ссылаться на ч.&л. |
uniform gas |
однородный газ |
averaged a |
усредненный |
3
exert v |
вызывать (напряжение), производить |
|
(давление) |
altitude n |
высота |
relative to |
относящийся к ч.&л.; по отношению к ч.&л. |
to be related to |
иметь отношение к ч&л. |
encounter v |
сталкиваться, встречаться |
fluid n |
жидкая среда |
fluid a |
текучий, газообразный, жидкий |
entire a |
весь, полный, целый |
ordered motion |
упорядоченное движение |
blast n |
прорыв |
net a |
общий, конечный |
angular momentum |
момент импульса; момент количества дви& |
|
жения |
viscosity n |
вязкость; тягучесть; внутреннее трение |
rotational a |
вихревой |
boundary layer |
пограничный слой |
drag n |
лобовое сопротивление, торможение |
compressibility n |
приходить в состояние |
to go into |
способность к сжатию, сжимаемость |
alter v |
изменять, переделывать |
shock wave |
ударная волна |
1. Find the transcriptions of the following words in a dictionary. Pronounce them carefully:
сharacteristics, characterize, proton, neutron, neon, oxygen, nitrogen, theory, diatomic, process, gas, through, location, rotational, macro, micro, kinetic, thermodynamic, lead, major, molecule.
2. Translate the following words and word combinations:
the air – the characteristics of air – the major components of air; the motion – the individual molecular motions – the large scale motion;
the property – the gas properties – the uniform gas properties.
4
3. Read and translate the text.
Text IA. Gas Properties Definitions
Aerodynamics involves the interactions between an object and the surrounding air. To better understand these interactions, we need to know some things about air.
Characteristics of Air
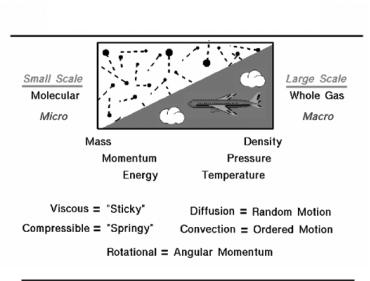
All matter is made from atoms with the configuration of the atom (number of protons, number of neutrons) determining the kind of matter present (oxygen, lead, silver, neon). Individual atoms can combine with other atoms to form molecules. In particular, oxygen and nitrogen, which are the major components of air, occur in nature as diatomic (2 atom) molecules. Under normal conditions, matter exists as either a solid, a liquid, or a gas. Air is a gas. In any gas, we have a very large number of molecules that are only weakly attracted to each other and are free to move about in space. When studying gases, we can investigate the motions and interactions of individual molecules, or we can investigate the large scale action of the gas as a whole. Scientists refer to the large scale motion of the gas as the macro scale and the individual molecular motions as the micro scale. Some phenomena are easier to understand and explain based on the macro scale, while other phenomena are more easily explained
5
on the micro scale. Macro scale investigations are based on things that we can easily observe and measure. But micro scale investigations are based on rather simple theories because we cannot actually observe an individual gas molecule in motion. Macro scale and micro scale investigations are just two views of the same thing.
Large Scale Motion of a Gas – Macro Scale
Air is treated as a uniform gas with properties that are averaged from all the individual components (oxygen, nitrogen, water vapor). On the macro scale, we are dealing with large scale effects that we can measure, such as the gas velocity, the pressure exerted on the surroundings, or the temperature of the gas. a gas does not have a fixed shape or size but will expand to fill any container. Because the molecules are free to move about in a gas, the mass of the gas is normally characterized by the density. On the macro scale, the properties of the gas can change with altitude and depend on the thermodynamic state of the gas. The state of the gas can be changed by thermodynamic processes.
Individual Molecular Motion of a Gas – Micro Scale
On the micro scale, air is modeled by the kinetic theory of gases. The model assumes that the molecules are very small relative to the distance between molecules. The molecules have the standard physical properties of mass, momentum, and energy. And these properties are related to the macro properties of density, pressure, and temperature. The interactions of the molecules introduce some other properties that we normally do not encounter when dealing with solids. In a solid, the location of the molecules relative to each other remains almost constant. But in a fluid, the molecules can move around and interact with each other and with their surroundings in different ways. As mentioned above, there is always a random component of molecular motion. But the entire fluid can be made to move as well in an ordered motion. As the molecules move, the properties of the fluid move as well. If the properties are transported by the random motion, the process is called diffusion. (an example of diffusion is the spread of an odor in a perfectly still room). If the properties are transported by the ordered motion, the process is called convection. (An example of convection is a blast of cold weather brought down from somewhere in the North.)
6
If the flow of a gas produces a net angular momentum, we say the flow is rotational. (No net angular momentum in the fluid is irrotational.)
Viscosity
As an object moves through the air, the viscosity (stickiness) of the air becomes very important. Air molecules stick to any surface, creating a layer of air near the surface (called a boundary layer) that, in effect, changes the shape of the object. To make things more confusing, the boundary layer may lift off or “separate” from the body and create an effective shape much different from the physical shape of an object. And to make it even more confusing, the flow conditions in and near the boundary layer are often unsteady (changing in time). The boundary layer is very important in determining both the drag and lift of an object.
Compressibility
As an object moves through the air, the compressibility of the air also becomes important. Air molecules move around an object as it passes through. If the object passes at a low speed (typically less than 200 mph), the density of the fluid remains constant. But for high speeds, some of the energy of the object goes into compressing the fluid, moving the molecules closer together and changing the air density, which alters the amount of the resulting force on the object. This effect is more important as speed increases. Near and beyond the speed of sound (about 700 mph), shock waves are produced that affect both the lift and drag of an object.
4.Answer the questions to the text.
1.What are the major components of air?
2.What states of substances can you come across in nature?
3.Why do scientists refer to the large scale motion of the gas as the macro scale and the individual molecular motions as the mi& cro scale?
4.What gas parameters can be measured?
5.What affects the gas properties?
6.Does gas have a fixed shape or size? Why?
7.When do we say the flow is rotational?
8.What effect do we have when the speed increases ?
9.What are the physical properties of the molecule?
7

5. Give the meanings of the words with the prefixes:
atomic–diatomic, action – interaction, to understand – to misunder& stand, normally – abnormally, to change – unchanged, steady – unsteady, rotational – irrotational, relative – non&relative, defined – undefined, moving – immoving, important – unimportant, compressibility – incompressibility.
6. Fill in the gaps with the words and word combinations from the box:
shock waves, molecules, lift, averaged, drag, kinetic theory, weakly attracted
1.As ________ move, the properties of the fluid move as well.
2.Near and beyond the speed of sound _____ are produced.
3.The boundary layer is very important in determining both ____
and _____ of an object.
4.On the micro scale air is modeled by ____ of gases.
5.In any gas we have a very large number of molecules that are only _____ to each other.
6.Air is treated as a uniform gas with properties that are _____
from all the individual components.
7.Complete the sentences using the information from the text.
1.Under normal conditions, matter exists _______.
2.Macro scale investigations are based on ______.
3.a gas does not have a fixed shape or size but _____.
4.The molecules have the standart physical properties of ______.
5.The state of the gas can be changed by ______.
8.Give the verbs in the brackets in the correct form.
1.Air (to be) a gas.
2.Matter (to exist) as either a solid, a liquid, or a gas.
3.Marco scale investigations (to be) based on things we can easily (to observe) and (to measure).
4.a gas does not (to have) a fixed shape or a size but (to expand) to fill any container.
5.We do not encounter other properties when (to deal) with solids.
8
6.(To make) things more confusing, the boundary layer (to lift) off or (to separate) from the body.
7.Air molecules move around the object as it (to pass) through.
8.Molecules are free (to move) about in a gas.
9.Say what parts of speech do the underlined words belong to. Translate them.
1.The mass of the gas is normally characterized by the density.
2.a gas does not have a fixed shape.
3.We are dealing with large scale effects that we can measure, such as the gas velocity, the pressure exerted on the surroundings.
4.As mentioned above, there is always a random component of mo& lecular motion.
5.Air molecules stick to any surface, creating a layer of air near the surface.
6.But for high speeds some of the energy of the object goes into compressing the fluid, moving molecules closer together and changing the air density.
7.When studying gases, we can investigate the motions and interactions of individual molecules.
10.Translate the sentences from Russian into English using the words from the text.
1.Изучая свойства газов, мы можем исследовать взаимодействие отдельных молекул.
2.Исследования наших ученых основываются на довольно простых теориях.
3.Беспорядочный поток жидкости можно заставить двигаться в заданном направлении.
4.Свойство вязкости воздуха ученые считают очень важным.
5.Если свойства газа переносятся в процессе упорядоченного движения молекул, то этот процесс называется конвекцией.
6.При скорости звука появляется ударная волна, которая влияет на подъемную силу объекта и на его лобовое сопро& тивление.
11.Read and translate the text using a dictionary if necessary.
9
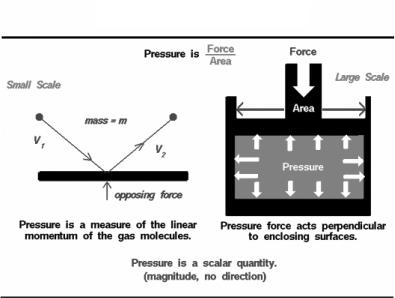
Text IB. Gas Pressure
An important property of any gas is its pressure. We have some experience with gas pressure that we don’t have with such properties like viscosity and compressibility. Every day we hear the TV meteorologist give value of the barometric pressure of the atmosphere (29.8 inches of mercury, for example). And most of us have blown up a balloon or used a pump to inflate a bicycle tire or a basketball.
There are two ways to look at pressure: (1) the small scale action of individual air molecules or (2) the large scale action of a large number of molecules.
Molecular Definition of Pressure
From the kinetic theory of gases, a gas is composed of a large number of molecules that are very small relative to the distance between molecules. The molecules of a gas are in constant, random motion and frequently collide with each other and with the walls of any container. The molecules pocess the physical properties of mass, momentum, and energy. The momentum of a single molecule is the product of its mass and velocity, while the kinetic energy is one half the mass times the square of the velocity. As the gas molecules collide with the walls of a container, as shown on the left of the figure, the molecules impart momentum to the walls, producing a force perpendicular to the wall. The sum of the forces of all the molecules striking the wall divided by the area of
10
