
187-2017
.pdfSaxagliptin is given orally as 2.5–5 mg daily. The drug reaches maximal concentrations within 2 hours (4 hours for its active metabolite). It is minimally protein bound and undergoes hepatic metabolism by CYP3A4/5. The major metabolite is active, and excretion is by both renal and hepatic pathways. The terminal plasma half-life is 2.5 hours for saxagliptin and 3.1 hours for its active metabolite. Dosage adjustment is recommended for individuals with renal impairment and concurrent use of strong CYP3A4/5 inhibitors such as antiviral, antifungal, and certain antibacterial agents. It is approved as monotherapy and in combination with biguanides, sulfonylureas, and thiazolidinediones. During clinical trials, monoand combination therapy with saxagliptin resulted in an HbA1c reduction of 0.4–0.9%.
Adverse effects include an increased rate of infections (upper respiratory tract and urinary tract), headaches, and hypersensitivity reactions (urticaria, facial edema). The dosage of a concurrently administered insulin secretagogue or insulin may need to be lowered to prevent hypoglycemia. Saxagliptin may increase the risk of heart failure. In a postmarketing study of 16,492 type 2 diabetes patients, there were 289 cases of heart failure in the saxagliptin group (3.5%) and 228 cases in the placebo group (2.8%)—a hazard ratio of 1.27. Patients at the highest risk for failure were those who had a history of heart failure or had elevated levels of N-terminal of the prohormone brain natriuretic peptide (NTpBNP) or had renal impairment.
Linagliptin lowers HbA1c by 0.4–0.6% when added to metformin, sulfonylurea, or pioglitazone. The dosage is 5 mg daily orally and, since it is primarily excreted via the bile, no dosage adjustment is needed in renal failure.
Adverse reactions include nasopharyngitis and hypersensitivity reactions (urticaria, angioedema, localized skin exfoliation, bronchial hyperreactivity). The risk of pancreatitis may be increased.
Alogliptin lowers HbA1c by about 0.5–0.6% when added to metformin, sulfonylurea, or pioglitazone. The usual dose is 25 mg orally daily. The 12.5-mg dose is used in patients with calculated creatinine clearance of 30 to 60 mL/min; the dose is 6.25 mg for clearance <30 mL/min. In clinical trials, pancreatitis occurred in 11 of 5902 patients on alogliptin (0.2%) and in 5 of 5183 patients receiving all comparators (<0.1%). There have been reports of hypersensitivity reactions (anaphylaxis, angioedema, StevensJohnson syndrome). Cases of hepatic failure have been reported, but it is unclear if alogliptin was the cause. The medication, however, should be discontinued in the event of liver failure.
Vildagliptin (not available in the United States) lowers HbA1c levels by 0.5–1% when added to the therapeutic regimen of patients with type 2 diabetes. The dosage is 50 mg orally once or twice daily. Adverse reactions include upper respiratory infections, nasopharyngitis, dizziness, and headache. Rarely, it can cause hepatitis, and liver function tests should be performed quarterly in the first year of use and periodically thereafter.
In animal studies, high doses of DPP-4 inhibitors and GLP-1 agonists cause expansion of pancreatic ductal glands and generation of premalignant pancreatic intraepithelial (PanIN) lesions that have the potential to progress to pancreatic adenocarcinoma. The relevance to human therapy is unclear and currently there is no evidence that these drugs cause pancreatic cancer in humans.
CHAPTER 41 Pancreatic Hormones & Antidiabetic Drugs |
763 |
SODIUM-GLUCOSE CO-TRANSPORTER 2 (SGLT2) INHIBITORS
Glucose is freely filtered by the renal glomeruli and is reabsorbed in the proximal tubules by the action of sodium-glucose transporters (SGLTs). Sodium-glucose transporter 2 (SGLT2) accounts for 90% of glucose reabsorption, and its inhibition causes glycosuria and lowers glucose levels in patients with type 2 diabetes. SGLT2 inhibitors lower glucose levels by changing the renal threshold and not by insulin action. The SGLT2 inhibitors canagliflozin, dapagliflozin, and empagliflozin, all oral medications, are approved for clinical use.
Canagliflozin reduces the threshold for glycosuria from a plasma glucose threshold of approximately 180 mg/dL to 70–90 mg/dL. It has been shown to reduce HbA1c by 0.6–1% when used alone or in combination with other oral agents or insulin. It also results in modest weight loss of 2–5 kg. The usual dosage is 100 mg daily. Increasing the dosage to 300 mg daily in patients with normal renal function can lower the HbA1c by an additional 0.5%.
Dapagliflozin reduces HbA1c by 0.5–0.8% when used alone or in combination with other oral agents or insulin. It also results in modest weight loss of about 2–4 kg. The usual dosage is 10 mg daily, but 5 mg daily is recommended initially in patients with hepatic failure.
Empagliflozin reduces HbA1c by 0.5–0.7% when used alone or in combination with other oral agents or insulin. It also results in modest weight loss of 2–3 kg. The usual dosage is 10 mg daily, but 25 mg/d may be used. In a postmarketing multinational study of 7020 type 2 patients with known cardiovascular disease, the addition of empagliflozin was associated with a lower primary composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (hazard ratio, 0.86; p = 0.04). The mechanisms regarding this benefit remain unclear. Weight loss, lower blood pressure, and diuresis may have played a role since there were fewer deaths from heart failure in the treated group whereas the rates of myocardial infarction were unaltered.
As might be expected, the efficacy of the SGLT2 inhibitors is reduced in chronic kidney disease. Canagliflozin and empagliflozin are contraindicated in patients with estimated GFR less than 45 mL/min per 1.73 m2. Dapagliflozin is not recommended for use in patients with estimated GFR less than 60 mL/min per 1.73 m2. The main adverse effects are increased incidence of genital infections and urinary tract infections affecting about 8–9% of patients. The osmotic diuresis can also cause intravascular volume contraction and hypotension. Canagliflozin and empagliflozin caused a modest increase in LDL cholesterol levels (4–8%). In clinical trials patients taking dapagliflozin had higher rates of breast cancer (nine cases versus none in comparator arms) and bladder cancer (nine cases versus one in placebo arm). These cancer rates exceeded the expected rates in an age-matched reference diabetes population. Canagliflozin has been reported to cause a decrease in bone mineral density at the lumbar spine and the hip. In a pooled analysis of 8 clinical trial (mean duration 68 weeks), an increase in fractures by about 30% was observed in patients
764 |
SECTION VII Endocrine Drugs |
on canagliflozin. It is likely that the effect on the bones is a class effect and not restricted to canagliflozin. A modest increase in upper limb fractures was observed with canagliflozin therapy. It is not known if this is due to an effect on bone strength or related to falls due to hypotension. Interim analysis of the Canagliflozin Cardiovascular Assessment Study clinical trial reported an approximately doubled risk of leg and foot amputations in the trial group assigned to Canagliflozin; in 2017 the FDA issued a drug safety communication regarding the association. Cases of diabetic ketoacidosis have been reported with off-label use of SGLT2 inhibitors in patients with type 1 diabetes. Type 1 patients are taught to give less insulin if their glucose levels are not elevated. Because type 1 patients on an SGLT2 inhibitor may have normal glucose levels, they may either withhold or reduce their insulin doses to such a degree as to induce ketoacidosis. Therefore, SGLT2 inhibitors should not be used in patients with type 1 diabetes and in those patients labelled as having type 2 diabetes but who are very insulin deficient and prone to ketosis.
OTHER HYPOGLYCEMIC DRUGS
Pramlintide is an islet amyloid polypeptide (IAPP, amylin) analog. IAPP is a 37-amino-acid peptide present in insulin secretory granules and secreted with insulin. It has approximately 46% homology with the calcitonin gene-related peptide (CGRP; see Chapter 17) and physiologically acts as a negative feedback on insulin secretion. At pharmacologic doses, IAPP reduces glucagon secretion, slows gastric emptying by a vagally mediated mechanism, and centrally decreases appetite. Pramlintide is an IAPP analog with substitutions of proline at positions 25, 28, and 29. These modifications make pramlintide soluble, non-self- aggregating, and suitable for pharmacologic use. Pramlintide is approved for use in insulin-treated type 1 and type 2 patients who are unable to achieve their target postprandial blood glucose levels. It is rapidly absorbed after subcutaneous administration; levels peak within 20 minutes, and the duration of action is not more than 150 minutes. It is metabolized and excreted by the kidney, but even at low creatinine clearance there is no significant change in bioavailability. It has not been evaluated in dialysis patients.
Pramlintide is injected immediately before eating; dosages range from 15 to 60 mcg subcutaneously for type 1 patients and from 60 to 120 mcg for type 2 patients. Therapy with this agent should be initiated at the lowest dosage and titrated upward. Because of the risk of hypoglycemia, concurrent rapidor shortacting mealtime insulin dosages should be decreased by 50% or more. Pramlintide should always be injected by itself using a separate syringe; it cannot be mixed with insulin. The major adverse effects of pramlintide are hypoglycemia and gastrointestinal symptoms, including nausea, vomiting, and anorexia. Since the drug slows gastric emptying, recovery from hypoglycemia can be problematic because of the delay in absorption of fast-acting carbohydrates.
Selected patients with type 1 diabetes who have problems with postprandial hyperglycemia can use pramlintide effectively to control the glucose rise especially in the setting of a high-carbohydrate
meal. The drug is not very useful in type 2 patients who can instead use the GLP-1 receptor agonists.
Colesevelam hydrochloride, the bile acid sequestrant and cholesterol-lowering drug, is approved as an antihyperglycemic therapy for persons with type 2 diabetes who are taking other medications or have not achieved adequate control with diet and exercise. The exact mechanism of action is unknown but presumed to involve an interruption of the enterohepatic circulation and a decrease in farnesoid X receptor (FXR) activation. FXR is a nuclear receptor with multiple effects on cholesterol, glucose, and bile acid metabolism. Bile acids are natural ligands of the FXR. Additionally, the drug may impair glucose absorption. In clinical trials, it lowered the HbA1c concentration 0.3–0.5%. Adverse effects include gastrointestinal complaints (constipation, indigestion, flatulence). It can also exacerbate the hypertriglyceridemia that commonly occurs in people with type 2 diabetes.
Bromocriptine, the dopamine agonist, in randomized placebocontrolled studies lowered HbA1c by 0–0.2% compared with baseline and by 0.4–0.5% compared with placebo. The mechanism by which it lowers glucose levels is not known.The main adverse events are nausea, fatigue, dizziness, vomiting, and headache.
Colesevelam and bromocriptine have very modest efficacy in lowering glucose levels, and their use for this purpose is questionable.
■ MANAGEMENT OF THE
PATIENT WITH DIABETES
Diet
A well-balanced, nutritious diet remains a fundamental element of therapy for diabetes. It is recommended that the macronutrient proportions (carbohydrate, protein, and fat) be individualized based on the patient’s eating patterns, preferences, and goals. Generally most patients with diabetes consume about 45% of their calories as carbohydrates; 25–35% fats; and 10–35% proteins. Limiting the carbohydrate intake and substituting some of the calories with monounsaturated fats, such as olive oil, rapeseed (canola) oil, or the oils in nuts and avocados, can lower triglycerides and increase HDL cholesterol. A Mediterranean-style eating pattern (a diet supplemented with walnuts, almonds, hazelnuts, and olive oil) has been shown to improve glycemic control and lower combined endpoints for cardiovascular events and stroke. Caloric restriction and weight loss is an important goal for the obese patient with type 2 diabetes.
Education
Education of the patient and family is a critical component of care. The patient should be informed about the kind of diabetes he or she has and the rationale for controlling the glucose levels (see Box: Benefits of Tight Glycemic Control in Diabetes). Selfmonitoring of glucose levels should be emphasized, especially if the patient is on insulin or oral secretagogues that can cause hypoglycemia. The patient on insulin therapy should understand

the action profile of the insulins. He or she should know how to determine if the basal insulin dose is correct and how to adjust the rapidly acting insulin dose for carbohydrate content of meals. Insulin adjustments for exercise and infections should be discussed. The patient and family members also should be informed about the signs and symptoms of hypoglycemia.
Glycemic Targets
The American Diabetes Association criteria for acceptable control include an HbA1c of less than 7% (53 mmol/mol) and pre-meal glucose levels of 90–130 mg/dL (5–7.2 mmol/L) and less than 180 mg/dL (10 mmol/L) one hour and 150 mg/dL (8.3 mmol/L) two hours after meals. While the HbA1c target is appropriate for individuals treated with lifestyle interventions and euglycemic therapy, it may need to be modified for individuals treated with insulin or insulin secretagogues due to their increased risk of hypoglycemia. Less stringent blood glucose control also is appropriate for children as well as patients with a history of severe hypoglycemia, limited life expectancy, and significant microvascular
CHAPTER 41 Pancreatic Hormones & Antidiabetic Drugs |
765 |
and macrovascular disease. For the elderly frail patient an HbA1c greater than 8% may be appropriate.
Treatment
Treatment must be individualized on the basis of the type of diabetes and specific needs of each patient.
A. Type 1 Diabetes
For most type 1 patients, at least 3 or 4 insulin injections a day are necessary for safe and effective control of glucose levels. A combination of rapidly acting insulin analogs and long-acting insulin analogs allow for more physiologic insulin replacement. Generally, for an adult with type 1 diabetes, the total daily insulin requirement in units is equal to the weight in pounds divided by four, or 0.55 times the person’s weight in kilograms. Approximately 40% of the total daily insulin dosage covers the background or basal insulin requirements, and the remainder covers meal and snack requirement and high blood sugar corrections. This is an approximate calculation and should be individualized. Examples of
Benefits of Tight Glycemic Control in Diabetes
A long-term randomized prospective study involving 1441 type 1 patients in 29 medical centers reported in 1993 that “near normalization” of blood glucose resulted in a delay in onset and a major slowing of progression of microvascular and neuropathic complications of diabetes during follow-up periods of up to 10 years (Diabetes Control and Complications Trial [DCCT] Research Group, 1993). In the intensively treated group, mean glycated hemoglobin (HbA1c) of 7.2% (normal <6%) and mean blood glucose of 155 mg/dL were achieved, whereas in the conventionally treated group, HbA1c averaged 8.9% with mean blood glucose of 225 mg/dL. Over the study period, which averaged 7 years, a reduction of approximately 60% in risk of diabetic retinopathy, nephropathy, and neuropathy was noted in the tight control group compared with the standard control group.
The DCCT study, in addition, introduced the concept of glycemic memory, which comprises the long-term benefits of any significant period of glycemic control. During a 6-year follow-up period, both the intensively and conventionally treated groups had similar levels of glycemic control, and both had progression of carotid intimal-medial thickness. However, the intensively treated cohort had significantly less progression of intimal thickness.
The United Kingdom Prospective Diabetes Study (UKPDS) was a very large randomized prospective study carried out to study the effects of intensive glycemic control with several types of therapies and the effects of blood pressure control in type 2 diabetic patients. A total of 3867 newly diagnosed type 2 diabetic patients were studied over 10 years. A significant fraction of these were overweight and hypertensive. Patients were given dietary treatment alone or intensive therapy with insulin, chlorpropamide, glyburide, or glipizide. Metformin was an option
for patients with inadequate response to other therapies. Tight control of blood pressure was added as a variable, with an angio- tensin-converting enzyme inhibitor, a β blocker, or in some cases, a calcium channel blocker available for this purpose.
Tight control of diabetes, with reduction of HbA1c from 9.1% to 7%, was shown to reduce the risk of microvascular complications overall compared with that achieved with conventional therapy (mostly diet alone, which decreased HbA1c to 7.9%). Cardiovascular complications were not noted for any particular therapy; metformin treatment alone reduced the risk of macrovascular disease (myocardial infarction, stroke). Epidemiologic analysis of the study suggested that every 1% decrease in the HbA1c achieved an estimated risk reduction of 37% for microvascular complications, 21% for any diabetes-related end point and death related to diabetes, and 14% for myocardial infarction.
Tight control of hypertension also had a surprisingly significant effect on microvascular disease (as well as more conventional hypertension-related sequelae) in these diabetic patients. Epidemiologic analysis of the results suggested that every 10-mmHg decrease in the systolic pressure achieved an estimated risk reduction of 13% for diabetic microvascular complications, 12% for any diabetes-related complication, 15% for death related to diabetes, and 11% for myocardial infarction.
Post-study monitoring showed that 5 years after the closure of the UKPDS, the benefits of intensive management on diabetic end points were maintained and the risk reduction for a myocardial infarction became significant. The benefits of metformin therapy were maintained.
These studies show that tight glycemic control benefits both type 1 and type 2 patients.

766 |
SECTION VII Endocrine Drugs |
TABLE 41 8 Examples of intensive insulin regimens using rapid-acting insulin analogs (insulin lispro, aspart, or glulisine) and NPH, or insulin detemir, glargine, or degludec in a 70-kg man with type 1 diabetes.1–3
|
Prebreakfast |
Prelunch |
Predinner |
Bedtime |
|
|
|
|
|
Rapid-acting insulin |
5 U |
4 U |
6 U |
— |
analog |
|
|
|
|
NPH insulin |
3 U |
3 U |
2 U |
8–9 U |
or |
|
|
|
|
Rapid-acting insulin |
5 U |
4 U |
6 U |
— |
analog |
|
|
|
|
Insulin glargine or |
— |
— |
— |
15–16 U |
degludec |
|
|
|
|
Insulin detemir |
6–7 U |
— |
— |
8–9 U |
|
|
|
|
|
1Assumes that patient is consuming approximately 75 g carbohydrate at breakfast, 60 g at lunch, and 90 g at dinner.
2The dose of rapid-acting insulin analogs can be raised by 1 or 2 U if extra carbohydrate (15–30 g) is ingested or if premeal blood glucose is >170 mg/dL. The rapid-acting insulin analogs can be mixed in the same syringe with NPH insulin.
3Insulin glargine or insulin detemir must be given as a separate injection.
reduced insulin requirement include newly diagnosed persons and those with ongoing endogenous insulin production, long-standing diabetes with insulin sensitivity, significant renal insufficiency, or other endocrine deficiencies. Increased insulin requirements typically occur with obesity, during adolescence, and during the latter trimesters of pregnancy. Table 41–8 illustrates regimens of rapidly acting insulin analogs and basal analogs that might be appropriate for a 70-kg person with type 1 diabetes. If the patient is on an insulin pump, he or she may require about a basal infusion rate of 0.6 units per hour throughout the 24 hours with the exception of 4:00 to 8:00 , when 0.7 units per hour might be appropriate (dawn phenomenon). The ratios might be one unit for 12 grams carbohydrate plus one unit for 50 mg/dL (2.8 mmol/L) of blood glucose above a target value of 120 mg/dL (6.7 mmol/L).
B. Type 2 Diabetes
Normalization of glucose levels can occur with weight loss and improvedinsulin sensitivity inthe obese patient with type 2 diabetes. A combination of caloric restriction and increased exercise is necessary if a weight reduction program is to be successful. Understanding the long-term consequences of poorly controlled diabetes may motivate some patients to lose weight. For selected patients, medical or surgical options should be considered. Orlistat, phentermine/ topiramate, lorcaserin, naltrexone plus extended release bupropion, and high-dose liraglutide are approved weight loss medications for use in combination with diet and exercise. Bariatric surgery (Roux-en-Y, gastric banding, gastric sleeve, biliopancreatic diversion/duodenal switch) typically result in significant weight loss and can result in remission of the diabetes.
Nonobese patients with type 2 diabetes frequently have increased visceral adiposity—the so-called metabolically obese normal weight patient. There is less emphasis on weight loss in such patients, but exercise is important.
Multiple medications may be required to achieve glycemic control (Figure 41–6) in patients with type 2 diabetes. Unless there is a contraindication, medical therapy should be initiated with
intensive lifestyle interventions (diet and exercise), diabetes selfmanagement education, and metformin. If clinical failure occurs with metformin monotherapy, a second agent is added. Options include sulfonylureas, repaglinide or nateglinide, pioglitazone, GLP-1 receptor agonists, DPP-4 inhibitors, SGLT2 inhibitors, and insulin. In the choice of the second agent, consideration should be given to efficacy of the agent, hypoglycemic risk, effect on weight, adverse effects, and cost. In patients who experience
Weight loss + exercise + metformin
*
Metformin + another agent
*
Metformin + two other agents
*
Metformin + more complex insulin regimen ± other non-insulin agent
*Step taken if needed to reach individualized HbA1c target after ~ 3 months.
Suggested algorithm for the treatment of type 2 diabetes. The seven main classes of agents are metformin, sulfonylureas (includes nateglinide, repaglinide), pioglitazone, GLP-1 receptor agonists, DPP-4 inhibitors, SGLT2 inhibitors, insulins. (α-Glucosidase inhibitors, colesevelam, pramlintide, and bromocriptine not included because of limited efficacy and significant adverse
reactions). (Data from the consensus panel of the American Diabetes Association/ European Association for the Study of Diabetes, as described in Inzucchi SE et al: Diabetes Care 2012;35:1364.)
hyperglycemia after a carbohydrate-rich meal (such as dinner), a short-acting secretagogue before that meal may suffice to control the glucose levels. Patients with severe insulin resistance may be candidates for pioglitazone. Patients who are very concerned about weight gain may benefit from a trial of a GLP-1 receptor agonist, a DPP-4 inhibitor, or an SGLT2 inhibitor, although the average weight loss with these medication is not great. If two agents are inadequate a third agent is added, although data regarding efficacy of such combined therapy are limited.
When the combination of oral agents and injectable GLP-1 receptor agonists fails to adequately control glucose levels, insulin therapy should be instituted. Various insulin regimens may be effective. Simply adding nighttime intermediateor long-acting insulin to the oral regimen may lead to improved fasting glucose levels and adequate control during the day. If daytime glucose levels are problematic, premixed insulins before breakfast and dinner may help. If such a regimen does not achieve adequate control or leads to unacceptable rates of hypoglycemia, a more intensive basal bolus insulin regimen (long-acting basal insulin) combined with rapid-acting analog before meals can be instituted. Metformin has been shown to be effective when combined with insulin therapy and should be continued. Pioglitazone can be used with insulin, but this combination is associated with more weight gain and peripheral and macular edema. Continuing with sulfonylureas, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT2 inhibitors can be of benefit in selected patients. Cost, complexity, and risk for adverse events should be considered when deciding which drugs to continue once the patient starts on insulin therapy.
Acute Complications of Diabetes
A. Hypoglycemia
Hypoglycemicreactionsarethe most commoncomplicationofinsulin therapy. It can also occur in any patient taking oral agents that stimulate insulin secretion (eg, sulfonylureas, meglitinide, -phenylalanine analogs), particularly if the patient is elderly, has renal or liver disease, or is taking certain other medications that alter metabolism of the sulfonylureas (eg, phenylbutazone, sulfonamides, warfarin). It occurs more frequently with the use of long-acting sulfonylureas.
Rapid development of hypoglycemia in persons with intact hypoglycemic awareness causes signs of autonomic hyperactivity— both sympathetic (tachycardia, palpitations, sweating, tremulousness) and parasympathetic (nausea, hunger)—and may progress to convulsions and coma if untreated.
In persons exposed to frequent hypoglycemic episodes during tight glycemic control, autonomic warning signals of hypoglycemia are less common or even absent. This dangerous acquired condition is termed hypoglycemic unawareness. When patients lack the early warning signs of low blood glucose, they may not take corrective measures in time. In patients with persistent, untreated hypoglycemia, the manifestations of insulin excess may develop— confusion, weakness, bizarre behavior, coma, seizures—at which point they may not be able to procure or safely swallow glucosecontaining foods. Hypoglycemic awareness may be restored by preventing frequent hypoglycemic episodes. An identification bracelet, necklace, or card in the wallet or purse, as well as some
CHAPTER 41 Pancreatic Hormones & Antidiabetic Drugs |
767 |
form of rapidly absorbed glucose, should be carried by every diabetic person who is receiving hypoglycemic drug therapy.
All the manifestations of hypoglycemia are relieved by glucose administration. To expedite absorption, simple sugar or glucose should be given, preferably in liquid form. To treat mild hypoglycemia in a patient who is conscious and able to swallow, dextrose tablets, glucose gel, or any sugar-containing beverage or food may be given. If more severe hypoglycemia has produced unconsciousness or stupor, the treatment of choice is 1 mg of glucagon injected either subcutaneously or intramuscularly. This may restore consciousness within 15 minutes to permit ingestion of sugar. Emergency medical services should be called in the event of loss of consciousness. The emergency personnel can restore consciousness by giving 20–50 mL of 50% glucose solution by intravenous bolus over a period of 2–3 minutes.
B. Diabetic Coma
1. Diabetic ketoacidosis—Diabetic ketoacidosis (DKA) is a life-threatening medical emergency caused by inadequate or absent insulin replacement, which occurs in people with type 1 diabetes and infrequently in those with type 2 diabetes. It typically occurs in newly diagnosed type 1 patients or in those who have experienced interrupted insulin replacement, and rarely in people with type 2 diabetes who have concurrent unusually stressful conditions such as sepsis or pancreatitis or are on highdose steroid therapy. DKA occurs more frequently in patients on insulin pumps. Poor compliance—either for psychological reasons or because of inadequate education—is one of the most common causes of DKA, particularly when episodes are recurrent.
Signs and symptoms include nausea, vomiting, abdominal pain, deep slow (Kussmaul) breathing, change in mental status (including coma), elevated blood and urinary ketones and glucose, an arterial blood pH lower than 7.3, and low bicarbonate (15 mmol/L).
The fundamental treatment for DKA includes aggressive intravenous hydration and insulin therapy and maintenance of potassium and other electrolyte levels. Fluid and insulin therapy is based on the patient’s individual needs and requires frequent reevaluation and modification. Close attention must be given to hydration and renal status, sodium and potassium levels, and the rate of correction of plasma glucose and plasma osmolality. Fluid therapy generally begins with normal saline. Regular human insulin should be used for intravenous therapy with a usual starting dosage of about 0.1 U/kg/h.
2. Hyperosmolar hyperglycemic syndrome—Hyperosmolar hyperglycemic syndrome (HHS) is diagnosed in persons with type 2 diabetes and is characterized by profound hyperglycemia and dehydration. It is associated with inadequate oral hydration, especially in elderly patients; with other illnesses; with the use of medication that elevates the blood sugar or causes dehydration, such as phenytoin, steroids, diuretics, and calcium channel blockers; and with peritoneal dialysis and hemodialysis. The diagnostic hallmarks are declining mental status and even seizures, a plasma glucose >600 mg/dL, and a calculated serum osmolality >320 mmol/L. Persons with HHS are not acidotic unless DKA is also present.
The treatment of HHS centers around aggressive rehydration and restoration of glucose and electrolyte homeostasis; the rate of
768 |
SECTION VII Endocrine Drugs |
correction of these variables must be monitored closely. Low-dose insulin therapy may be required.
Chronic Complications of Diabetes
Late clinical manifestations of diabetes mellitus include a number of pathologic changes that involve small and large blood vessels, cranial and peripheral nerves, the skin, and the lens of the eye.These lesions lead to hypertension, end-stage chronic kidney disease, blindness, autonomic and peripheral neuropathy, amputations of the lower extremities, myocardial infarction, and cerebrovascular accidents. These late manifestations correlate with the duration of the diabetic
state subsequent to the onset of puberty and glycemic control. In type 1 diabetes, end-stage chronic kidney disease develops in up to 40% of patients, compared with less than 20% of patients with type 2 diabetes. Proliferative retinopathy ultimately develops in both types of diabetes but has a slightly higher prevalence in type 1 patients (25% after 15 years’ duration). In patients with type 1 diabetes, complications from end-stage chronic kidney disease are a major cause of death, whereas patients with type 2 diabetes are more likely to have macrovascular diseases leading to myocardial infarction and stroke as the main causes of death. Cigarette use adds significantly to the risk of both microvascular and macrovascular complications in diabetic patients.
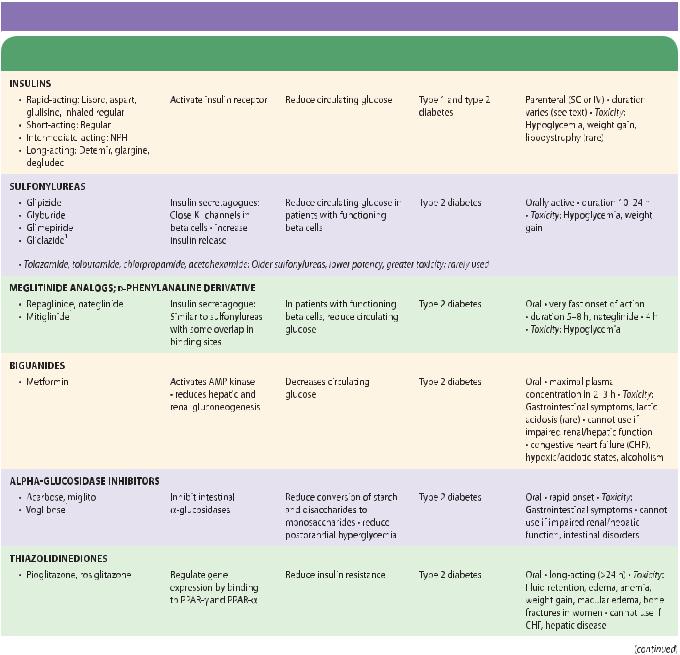
SUMMARY Drugs Used for Diabetes
|
Mechanism of |
|
Clinical |
Pharmacokinetics, |
Subclass, Drug |
Action |
Effects |
Applications |
Toxicities, Interactions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|
|
CHAPTER 41 |
Pancreatic Hormones & Antidiabetic Drugs |
769 |
|||
|
Mechanism of |
|
|
Clinical |
|
Pharmacokinetics, |
|
|
|
|
|
|
|||
Subclass, Drug |
Action |
Effects |
|
Applications |
|
Toxicities, Interactions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1Not available in United States.

770 SECTION VII Endocrine Drugs
P R E P A R A T I O N S A V A I L A B L E*
|
|
|
|
|
|
GENERIC NAME |
AVAILABLE AS |
GENERIC NAME |
AVAILABLE AS |
||
SULFONYLUREAS |
THIAZOLIDINEDIONE COMBINATION |
||||
Acetohexamide‡ |
Generic, Dymelor |
Pioglitazone plus glimepiride |
Duetact |
||
Chlorpropamide |
Generic, Diabinese |
Alogliptin plus pioglitazone |
Oseni |
||
Gliclazide‡ |
Generic, Diamicron |
Rosiglitazone plus glimepiride |
Avandaryl |
||
Glimepiride |
Generic, Amaryl |
ALPHA-GLUCOSIDASE INHIBITORS |
|||
Glipizide |
Generic, Glucotrol, Glucotrol XL |
Acarbose |
Generic, Precose |
||
Glyburide |
Generic, Diaβeta, Micronase, |
Miglitol |
Glyset |
||
|
Glynase PresTab |
Voglibose‡ |
|
|
|
Tolazamide |
Generic, Tolinase |
GLUCAGON-LIKE POLYPEPTIDE-1 RECEPTOR AGONISTS |
|||
Tolbutamide |
Generic, Orinase |
Exenatide |
Byetta |
||
MEGLITINIDES |
Liraglutide |
Victoza |
|||
Repaglinide |
Generic, Prandin |
Albiglutide |
Tanzeum, Eperzan |
||
Mitiglinide‡ |
|
Dulaglutide |
Trulicity |
||
D-PHENYLALANINE DERIVATIVE |
DIPEPTIDYL PEPTIDASE-4 INHIBITORS |
||||
Nateglinide |
Generic, Starlix |
Linagliptin |
Tradjenta |
||
BIGUANIDE |
Saxagliptin |
Onglyza |
|||
Metformin |
Generic, Glucophage, |
Sitagliptin |
Januvia |
||
|
Glucophage XR |
Alogliptin |
Nesina |
||
METFORMIN COMBINATIONS† |
|||||
‡ |
|
|
|||
Glipizide plus metformin |
Generic, Metaglip |
Vildagliptin |
|
|
|
SODIUM GLUCOSE CO-TRANSPORTER 2 INHIBITORS |
|||||
Glyburide plus metformin |
Generic, Glucovance |
||||
Canagliflozin |
Invokana |
||||
Pioglitazone plus metformin |
ACTOplus Met |
||||
Dapagliflozin |
Farxiga |
||||
Repaglinide plus metformin |
Prandi-Met |
||||
Empagliflozin |
Jardiance |
||||
Rosiglitazone plus metformin |
Avandamet |
||||
SODIUM GLUCOSE CO-TRANSPORTER |
|||||
Saxagliptin plus metformin |
Kombiglyze |
||||
INHIBITORS COMBINATION |
|||||
Sitagliptin plus metformin |
Janumet |
Empagliflozin plus linagliptin |
Glyxambi |
||
Linagliptin plus metformin |
Jentadueto |
ISMISCELLANEOUS DRUGSLET AMYLOID POLYPEPTIDE ANALOG |
|||
Alogliptin plus metformin |
Kazano |
Pramlintide |
Symlin |
||
Dapagliflozin plus metformin |
Xigduo |
BILE ACID SEQUESTRANT |
|||
Canagliflozin plus metformin |
Invokamet |
Colesevelam hydrochloride |
Welchol |
||
Empagliflozin plus metformin |
Synjardy |
DOPAMINE RECEPTOR AGONIST |
|||
THIAZOLIDINEDIONE DERIVATIVES |
Bromocriptine |
Generic, Parlodel, Cycloset |
|||
Pioglitazone |
Generic, Actos |
GLUCAGON |
|||
Rosiglitazone |
Avandia |
Glucagon |
Generic |
||
|
|
|
|
|
|
See Table 41–5 for insulin preparations.
†Other combinations are available.
‡Not available in the United States.
REFERENCES
Action to Control Cardiovascular Risks in Diabetes Study Group: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545.
Adler AI et al: Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. Br Med J 2000;321:412.
ADVANCE Collaborative Group: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560.
Ahmadian M et al: PPARγ signaling and metabolism: The good, the bad and the future. Nat Med 2013;19:557.
American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36(Suppl 1):S67.
Andrianesis V, Doupis J: The role of kidney in glucose homeostasis—SGLT2 inhibitors, a new approach in diabetes treatment. Expert Rev Clin Pharmacol 2013;6:519.
Bennett WL et al: Comparative effectiveness and safety of medications for type 2 diabetes: An update including new drugs and 2-drug combinations. Ann Intern Med 2011;154:602. Erratum in: Ann Intern Med 2011;155:67.
Butler PC et al: A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe? Diabetes Care 2013;36:2118.
Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393.
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl 1):S5.

Gaede P et al: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580.
Guo S: Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models to disease mechanisms. J Endocrinol 2014;220:T1.
Inzucchi SE et al: Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015;58:429.
Karagiannis T et al: Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: Systematic review and meta-analysis. BMJ 2012;344:e1369.
Kitabchi A et al: Thirty years of personal experience in hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab 2008;93:1541.
Lefebvre P et al: Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009;89:147.
Levin D et al: Pioglitazone and bladder cancer risk: A multipopulation pooled, cumulative exposure analysis. Diabetologia 2015;58:493.
Miyazaki Y, DeFronzo RA: Rosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in type 2 diabetic patients. Diabetes Obes Metab 2008;10:1204.
Nauck M: Incretin therapies: Highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2016;18:203.
Nwose OM, Jones MR: Atypical mechanism of glucose modulation by colesevelam in patients with type 2 diabetes. Clin Med Insights Endocrinol Diabetes 2013;6:75.
CHAPTER 41 Pancreatic Hormones & Antidiabetic Drugs |
771 |
Ratner RE et al: Amylin replacement with pramlintide as an adjunct to insulin therapy improves long term glycemic and weight control in type 1 diabetes mellitus: A 1-year randomized controlled trial. Diabetic Med 2004; 21:1204.
Reitman ML et al: Pharmacogenetics of metformin response: A step in the path toward personalized medicine. J Clin Invest 2007;117:1226.
Rizzo M et al: Non-glycemic effects of pioglitazone and incretin-based therapies. Expert Opin Ther Targets 2013;17:739.
Rosenstock J, Ferrannini E: Euglycemic diabetic ketoacidosis: A predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015;38:1638.
Standl E, Schnell O: Alpha-glucosidase inhibitors 2012—cardiovascular considerations and trial evaluation. Diab Vasc Dis Res 2012;9:163.
Switzer SM et al: Intensive insulin therapy in patients with type 1 diabetes mellitus. Endocrinol Metab Clin North Am 2012;41:89.
Tuccori M et al: Pioglitazone use and risk of bladder cancer: population based cohort study. BMJ 2016;352:i1541.
United Kingdom Prospective Diabetes Study (UKPDS) Group: Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies: UKPDS 49. JAMA 1999;281:2005.
United Kingdom Prospective Diabetes Study (UKPDS) Group: Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703.
C A S E S T U D Y A N S W E R
This patient had significant insulin resistance, taking about 125 units of insulin daily (approximately 1 unit per kilogram). He had had limited instruction on how to manage his diabetes. He had peripheral neuropathy, proteinuria, low HDL cholesterol levels, and hypertension. The patient underwent multifactorial intervention targeting his weight, glucose levels, and blood pressure. He was advised to stop smoking. He attended structured diabetes classes and received individualized instruction from a diabetes educator and a dietitian. Metformin therapy was reinitiated and his insulin doses were reduced. The patient was then given the GLP1 receptor
agonist, exenatide. The patient lost about 8 kg in weight over the next 3 years and was able to stop his insulin. He had excellent control with an HbA1c of 6.5 % on a combination of metformin, exenatide, and glimepiride. His antihypertensive therapy was optimized and his urine albumin excretion declined to 1569 mg/g creatinine. This case illustrates the importance of weight loss in controlling glucose levels in the obese patient with type 2 diabetes. It also shows that simply increasing the insulin dose is not always effective. Combining metformin with other oral agents and non-insulin injectables may be a better option.

C H A P T E R
Agents That Affect Bone
Mineral Homeostasis
Daniel D. Bikle, MD, PhD
C A S E S T U D Y
A 65-year-old man is referred to you from his primary care physician (PCP) for evaluation and management of possible osteoporosis. He saw his PCP for evaluation of low back pain. X-rays of the spine showed some degenerative changes in the lumbar spine plus several wedge deformities in the thoracic spine. The patient is a long-time smoker (up to two packs per day) and has two to four glasses of wine with dinner, more on the weekends. He has chronic bronchitis, presumably from smoking, and has been treated on numerous occasions with oral prednisone for exacerbations of bronchitis. He is currently on 10 mg/d prednisone.
■ BASIC PHARMACOLOGY
Calcium and phosphate, the major mineral constituents of bone, are also two of the most important minerals for general cellular function. Accordingly, the body has evolved complex mechanisms to carefully maintain calcium and phosphate homeostasis (Figure 42–1). Approximately 98% of the 1–2 kg of calcium and 85% of the 1 kg of phosphorus in the human adult are found in bone, the principal reservoir for these minerals. This reservoir is dynamic, with constant remodeling of bone and ready exchange of bone mineral with that in the extracellular fluid. Bone also serves as the principal structural support for the body and provides the space for hematopoiesis. This relationship is more than fortuitous, as elements of the bone marrow affect skeletal processes just as skeletal elements affect hematopoietic processes. During aging and in nutritional diseases such as anorexia nervosa and obesity, fat accumulates in the marrow, suggesting a dynamic interaction between marrow fat and bone. Furthermore, bone has
Examination shows kyphosis of the thoracic spine, with some tenderness to fist percussion over the thoracic spine. The dual-energy x-ray absorptiometry (DEXA) measurement of the lumbar spine is “within the normal limits,” but the radiologist noted that the reading may be misleading because of degenerative changes. The hip measurement shows a T score (number of standard deviations by which the patient’s measured bone density differs from that of a normal young adult) in the femoral neck of –2.2. What further workup should be considered, and what therapy should be initiated?
been implicated as an endocrine tissue with release of osteocalcin, which in its uncarboxylated form stimulates insulin secretion and testicular function. Abnormalities in bone mineral homeostasis can lead to a wide variety of cellular dysfunctions (eg, tetany, coma, muscle weakness), disturbances in structural support of the body (eg, osteoporosis with fractures), and loss of hematopoietic capacity (eg, infantile osteopetrosis).
Calcium and phosphate enter the body from the intestine. The average American diet provides 600–1000 mg of calcium per day, of which approximately 100–250 mg is absorbed. This amount represents net absorption, because both absorption (principally in the duodenum and upper jejunum) and secretion (principally in the ileum) occur. The quantity of phosphorus in the American diet is about the same as that of calcium. However, the efficiency of absorption (principally in the jejunum) is greater, ranging from 70% to 90%, depending on intake. In the steady state, renal excretion of calcium and phosphate balances intestinal absorption. In general, more than 98% of filtered calcium and 85% of
772
