
- •Internal combustion engine
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Using the English-Russian dictionary translate the following text
- •Into Russian:
- •IV. Translate the following information into English:
- •Translate the following sentences into Russian:
- •Explain and translate the following definitions of the car body elements:
- •Translate the following information into English:
- •Translate the following information into Russian:
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Translate the following information into English:
- •Translate the following text into Russian:
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate the following text into Russian:
- •Be ready to talk and discuss the general structure of a ship
- •Uss Nimitz’s catapult 1
- •Translate the following text into English:
- •Inspecting a used car
- •Incandescent lighting - освещение лампами накаливания
- •Answer and translate the following questions:
- •Translate the following sentences:
- •Translate the following text into Russian:
- •IV. Translate the following information into English:
- •Various definitions
- •Decipher and translate the following abbreviations:
- •Answer the following question:
- •Translate the following text into Russian:
- •Translate the following material into English:
- •I. Translate the following sentences:
- •II. Translate the following information in to Russian:
- •Translate the following advertising material into English:
- •Be ready to talk about the power supply at your home.
- •Introduction to power electronics
- •I. Answer the following questions:
- •Translate into English words in brackets and then the sentences
- •Into Russian:
- •Translate the following text: Conductors
- •Translate the following sentences:
- •Translate into English: Теория цепей
- •Translate into Russian: Current supply
- •Give equivalents to the following words and word combinations:
- •Translate and answer the following questions:
- •Translate the following text:
- •Translate the text and be ready to discuss the general radio design:
- •Translate into English the following material:
- •Translate the following extract: Microphone transmitter.
- •Introduction to radar fundamentals
- •Give equivalents to the following words and word combinations:
- •Put 10 questions to the text and answer them:
- •Translate into Russian the following text:
- •Translate into English:
- •Read and translate the list of chemical elements with their symbols and atomic number: (in alphabetical order)
- •Translate the following sentences:
- •Translate into Russian:
- •Translate into English:
- •Translate the following information into English:
- •Put 6 questions to the text and answer them:
- •Translate the following sentences in to Russian:
- •Translate the following text and he ready to discuses properties of elements: Chemical properties of elements
- •Vanderwaals radius
- •Ionic radius
- •Isotopes
- •Translate the following text into English using proper terms given below:
- •Classification by Structural Change
- •Classification by Reaction Type
- •Translate into Russian the following sentences:
- •Translate the follow text into Russian:
- •Translate the classification of hydrocarbons into Russian:
- •Reaction Characteristics
- •Factors that Influence Reactions
- •Translate the following information into Russian:
- •Translate the following text into English: Насыщенные углеводороды
- •Translate the following material into Russian:
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate into English:
- •Translate the following text into Russian:
- •Translate the following information into English:
- •Translate the words and word combinations in brackets into English.
- •Give equivalents to the following word combinations:
- •Translate the following text into Russian:
- •Translate into English:
- •Translate into Russian:
- •Be ready to answer questions on the text and talk about the refinery presses.
- •Translate words and word combinations in brackets into English. Translate
- •Give equivalents to the following word combinations:
- •Translate the following text:
- •Translate into English:
- •Translate into Russian:
- •Translate into English:
- •Translate in to English:
- •Interface поверхность раздела; граница
- •Viscosity
- •Translate words and word combinations in brackets into English. Then
- •Translate the following text and be ready to discuss it: crude oil pretreatment (desalting).
- •Electrostatic desalting.
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate the following text into English:
- •Translate the following information into English:
- •Give eqvivalents to the following words and word combinations:
- •Answer the following questions:
- •Translate the following sentences:
- •Translate the following text into English:
- •Translate the following text:
- •Translate into English:
- •Improving the quality of petroleum products
- •Give eqvivalents to the following words and word combinations:
- •Answer the following questions:
- •Translate into Russian:
- •Translate into English:
- •Translate into Russian: The Physics of Oil Refineries
- •Discussion of the refinery process. Prepare the brief report on one of the theme topics.
- •Translate the following sentences:
- •Translate the following text in writing:
- •Introduction to nuclear power
- •Translate the following sentences into Russian:
- •Translate the following text into Russian:
- •Translate the following text into English:
- •Inside the reactor
- •Put some questions to the text and translate them:
- •Translate the following sentences:
- •Translate the following material into English:
- •Translate the following text into Russian:
- •Translate the following text into Russian:
- •Translate the following word combination into Russian:
- •Translate the following sentences into Russian:
- •Translate the following text into English:
- •Translate the following text into English:
- •Translate the following extract into English:
- •Translate the following sentences:
- •Translate the following information into Russian:
- •Translate into Russian:
- •Translate the following information into English:
- •Translate the following information into English:
- •Give equivalents to the terms on the fig.2 using technical dictionaries.
- •Translate into Russian:
- •Translate into Russian:
- •Translate the following information into Russian:
- •Translate into English: Устройство энергетических ядерных реакторов.
- •Put several questions to the text above and answer them:
- •Translate the following text into Russian:
- •Translate the following extract into Russian:
- •To check yourself try to translate the following text at sight:
- •Introduction to the almr/prism
- •Translate the following information into English: хранение ядерного топлива
- •Work area
- •Electrical safety
- •Power tool use and care
- •Service
- •Рабочее место
- •Поддерживайте чистоту и порядок на Вашем рабочем месте.
- •Меры безопасности при подключении к электросети.
- •Указания по безопасности
- •Использование инструмента и уход за ним.
- •Important safety instructions
- •Translate into English:
- •Устройство и принцип работы р ис. 1. Внешний вид аппарата.
- •6 Claims, 5 Drawing Figures exhaust gas recirculation apparatus for engine with turbocharger
- •Read the patent given above, identify its parts and be ready to comment on peculiarities of their translation. 2. Translate the following extract into English:
- •Beltline: The horizontal area of the body along the door just below the side-window glass.
- •Abstract
- •Description
Translate the following sentences into Russian:
In the fission, the nucleus of a heavy element, such as uranium, when bombarded by a free neutron splits into two smaller atoms.
In nuclear power plants, heat energy generated by burning uranium fuel is collected in ordinary water that drives a turbine to produce electrical power.
In a pressurized-water reactor, the superheated water in the primary cooling loop is used to transfer heat energy to a secondary loop for the creation of steam.
In other reactor designs, the heat energy is transferred by pressurized cooling substance such as gas, nitrogen or heavy water.
The different levels of enrichment required for a particular nuclear reactor are specified by the customer: light-water reactor 23 5U normally is enriched up to about 4 percent.
Gaseous diffusion and gas centrifuge are the commonly used uranium enrichment technologies.
The pellets are then fired in a high temperature sintering furnace to create hard, ceramic pellets of enriched uranium.
When the reactor is shut down for refueling the spent fuel discharged at that time is stored either at the reactor site or, potentially, in a common facility away from reactor sites.
The recovered uranium and plutonium can, if economic and institutional conditions permit, be recycled for use as nuclear fuel.
Translate the following text into Russian:
We know that certain elements are constantly and naturally on the verge of instability and being unstable these elements radiate particles from their nuclei. They require no outside stimulus to make them throw out particles. This does not mean that every atom of elements such as radium throw out particles at the instant they are formed. They may be just on the verge of instability, and as the atom moves about in constant vibration, the particles in the nucleus may shift their positions just enough to make the forces of repulsion overcome those of attraction and throw out a particle or two.
But it’s a matter of chance. One particular atom of radium may not disintegrate for a billion of years, while another may disintegrate in a small fraction.
In any visible piece of radium, however, there are so many countless atoms that the average always holds true. So we can say that the half-life of radium 266 is 1,590 years. This means that within this period, one-half of atoms in any piece of radium will have disintegrated through natural radioactivity.
Translate the following text into English:
Радиоактивность.
Радиоактивность — это испускание ядрами некоторых элементов различных частиц, сопровождающееся переходом ядра в другое состояние и изменением его параметров. Явление радиоактивности было открыто опытным путем французским ученым Анри Беккерелем в 1896 г. для солей урана. Беккерель заметил, что соли урана засвечивают завернутую во много слоев фотобумагу невидимым проникающим излучением.
А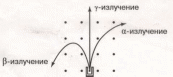 нглийский
физик Э. Резерфорд исследовал
радиоактивное излучение в электрических
и магнитных полях и открыл три составляющие
этого излучения, которые были названы
а-, В-, у- излучением (рис. 1).
нглийский
физик Э. Резерфорд исследовал
радиоактивное излучение в электрических
и магнитных полях и открыл три составляющие
этого излучения, которые были названы
а-, В-, у- излучением (рис. 1).
а-Распад представляет собой излучение а-частиц (ядер
гелия) высоких энергий. При этом масса ядра уменьшается на 4 единицы, а заряд
на 2 единицы.
Р-Распад — излучение электронов, заряд которых возрастает на единицу, массовое число не изменяется.
у-Излучение представляет собой испускание возбужденным ядром квантов света высокой частоты. Параметры ядра при у-излучении не меняются, ядро лишь переходит в состояние с меньшей энергией. Распавшееся ядро тоже радиоактивно, т. е. происходит цепочка последовательных радиоактивных превращений. Процесс распада всех радиоактивных элементов идет до свинца. Свинец — конечный продукт распада.
Приборы, применяемые для регистрации ядерных излучений, называются детекторами ядерных излучений.
Радиоактивные излучения оказывают сильное биологическое действие на ткани живого организма, заключающееся в ионизации атомов и молекул среды. Большие дозы облучения приводят к смерти.
Translate the following text: Ядерный топливный цикл.
Атомная энергетика - это сложное производство, включающее множество промышленных процессов, которые вместе образуют топливный цикл. Существуют различные типы топливных циклов, зависящие от типа реактора.
Обычно топливный цикл состоит из следующих процессов. В рудниках добывается урановая руда. Руда измельчается для отделения диоксида урана, а радиоактивные отходы идут в отвал. Полученный оксид урана (желтый кекс) преобразуется в гексафторид урана - газообразное соединение. Для повышения концентрации урана-235 гексафторид урана обогащают на заводах по разделению изотопов. Затем обогащенный уран снова переводят в твердый диоксид урана, из которого изготавливают топливные таблетки. Из таблеток собирают тепловыделяющие элементы (Твэлы), которые объединяют в сборки для ввода в активную зону ядерного реактора АЭС.
LESSON # 2
WHAT IS URANIUM?
Uranium is a very heavy (dense) metal which can be used as an abundant source of concentrated energy.
It occurs in most rocks in concentrations of 2 to 4 parts per million and is as common in the earth's crust as tin, tungsten and molybdenum. It occurs in seawater, and could be recovered from the oceans if prices rose significantly.
It was discovered in 1789 by Martin Klaproth, a German chemist, in the mineral called pitchblende. It was named after the planet Uranus, which had been discovered eight years earlier.
Uranium was apparently formed in super novae about 6.6 billion years ago. While it is not common in the solar system, today its radioactive decay provides the main source of heat inside the earth, causing convection and continental drift.
The high density of uranium means that it also finds uses in the keels of yachts and as counterweights for aircraft control surfaces (rudders and elevators), as well as for radiation shielding.
Its melting point is 1132°C. The chemical symbol for uranium is U.
The Uranium Atom. On a scale arranged according to the increasing mass of their nuclei, uranium is the heaviest of all the naturally-occurring elements (Hydrogen is the lightest). Uranium is 18.7 times as dense as water. Like other elements, uranium occurs in slightly differing forms known as ‘isotopes’. These isotopes (16 in the case of uranium) differ from each other in the number of particles (neutrons) in the nucleus. ‘Natural’ uranium as found in the earth’s crust is a mixture largely of two isotopes: uranium-238 (U-238), accounting for 99.3% and U-235 about 0.7%.
The isotope U-235 is important because under certain conditions it can readily be split, yielding a lot of energy. It is therefore said to be ‘fissile’ and we use the expression ‘nuclear fission’
Meanwhile, like all radioactive isotopes, it decays. U-238 decays very slowly, its half-life being the same as the age of the earth (4500 million years). This means that it is barely radioactive, less so than many other isotopes in rocks and sand. Nevertheless it generates 0.1 watts/tonne and this is enough to warm the earth’s core.
Energy from the uranium atom. The nucleus of the U-235 atom comprises 92 protons and 143 neutrons (92 + 143 = 235). When the nucleus of a U-235 atom captures a neutron it splits in two (fissions) and releases some energy in the form of heat, also two
or three additional neutrons are thrown off. If enough of these expelled neutrons cause the nuclei of other U-235 atoms to split, releasing further neutrons, fission ‘chain reac- tion№ can be achieved. When this happens over and over again, many millions of times, a very large amount of heat is produced from a relatively small amount of uranium.
It is this process, in effect “burning” uranium, which occurs in a nuclear reactor. The heat is used to make steam to produce electricity.
