
- •Internal combustion engine
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Using the English-Russian dictionary translate the following text
- •Into Russian:
- •IV. Translate the following information into English:
- •Translate the following sentences into Russian:
- •Explain and translate the following definitions of the car body elements:
- •Translate the following information into English:
- •Translate the following information into Russian:
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Translate the following information into English:
- •Translate the following text into Russian:
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate the following text into Russian:
- •Be ready to talk and discuss the general structure of a ship
- •Uss Nimitz’s catapult 1
- •Translate the following text into English:
- •Inspecting a used car
- •Incandescent lighting - освещение лампами накаливания
- •Answer and translate the following questions:
- •Translate the following sentences:
- •Translate the following text into Russian:
- •IV. Translate the following information into English:
- •Various definitions
- •Decipher and translate the following abbreviations:
- •Answer the following question:
- •Translate the following text into Russian:
- •Translate the following material into English:
- •I. Translate the following sentences:
- •II. Translate the following information in to Russian:
- •Translate the following advertising material into English:
- •Be ready to talk about the power supply at your home.
- •Introduction to power electronics
- •I. Answer the following questions:
- •Translate into English words in brackets and then the sentences
- •Into Russian:
- •Translate the following text: Conductors
- •Translate the following sentences:
- •Translate into English: Теория цепей
- •Translate into Russian: Current supply
- •Give equivalents to the following words and word combinations:
- •Translate and answer the following questions:
- •Translate the following text:
- •Translate the text and be ready to discuss the general radio design:
- •Translate into English the following material:
- •Translate the following extract: Microphone transmitter.
- •Introduction to radar fundamentals
- •Give equivalents to the following words and word combinations:
- •Put 10 questions to the text and answer them:
- •Translate into Russian the following text:
- •Translate into English:
- •Read and translate the list of chemical elements with their symbols and atomic number: (in alphabetical order)
- •Translate the following sentences:
- •Translate into Russian:
- •Translate into English:
- •Translate the following information into English:
- •Put 6 questions to the text and answer them:
- •Translate the following sentences in to Russian:
- •Translate the following text and he ready to discuses properties of elements: Chemical properties of elements
- •Vanderwaals radius
- •Ionic radius
- •Isotopes
- •Translate the following text into English using proper terms given below:
- •Classification by Structural Change
- •Classification by Reaction Type
- •Translate into Russian the following sentences:
- •Translate the follow text into Russian:
- •Translate the classification of hydrocarbons into Russian:
- •Reaction Characteristics
- •Factors that Influence Reactions
- •Translate the following information into Russian:
- •Translate the following text into English: Насыщенные углеводороды
- •Translate the following material into Russian:
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate into English:
- •Translate the following text into Russian:
- •Translate the following information into English:
- •Translate the words and word combinations in brackets into English.
- •Give equivalents to the following word combinations:
- •Translate the following text into Russian:
- •Translate into English:
- •Translate into Russian:
- •Be ready to answer questions on the text and talk about the refinery presses.
- •Translate words and word combinations in brackets into English. Translate
- •Give equivalents to the following word combinations:
- •Translate the following text:
- •Translate into English:
- •Translate into Russian:
- •Translate into English:
- •Translate in to English:
- •Interface поверхность раздела; граница
- •Viscosity
- •Translate words and word combinations in brackets into English. Then
- •Translate the following text and be ready to discuss it: crude oil pretreatment (desalting).
- •Electrostatic desalting.
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate the following text into English:
- •Translate the following information into English:
- •Give eqvivalents to the following words and word combinations:
- •Answer the following questions:
- •Translate the following sentences:
- •Translate the following text into English:
- •Translate the following text:
- •Translate into English:
- •Improving the quality of petroleum products
- •Give eqvivalents to the following words and word combinations:
- •Answer the following questions:
- •Translate into Russian:
- •Translate into English:
- •Translate into Russian: The Physics of Oil Refineries
- •Discussion of the refinery process. Prepare the brief report on one of the theme topics.
- •Translate the following sentences:
- •Translate the following text in writing:
- •Introduction to nuclear power
- •Translate the following sentences into Russian:
- •Translate the following text into Russian:
- •Translate the following text into English:
- •Inside the reactor
- •Put some questions to the text and translate them:
- •Translate the following sentences:
- •Translate the following material into English:
- •Translate the following text into Russian:
- •Translate the following text into Russian:
- •Translate the following word combination into Russian:
- •Translate the following sentences into Russian:
- •Translate the following text into English:
- •Translate the following text into English:
- •Translate the following extract into English:
- •Translate the following sentences:
- •Translate the following information into Russian:
- •Translate into Russian:
- •Translate the following information into English:
- •Translate the following information into English:
- •Give equivalents to the terms on the fig.2 using technical dictionaries.
- •Translate into Russian:
- •Translate into Russian:
- •Translate the following information into Russian:
- •Translate into English: Устройство энергетических ядерных реакторов.
- •Put several questions to the text above and answer them:
- •Translate the following text into Russian:
- •Translate the following extract into Russian:
- •To check yourself try to translate the following text at sight:
- •Introduction to the almr/prism
- •Translate the following information into English: хранение ядерного топлива
- •Work area
- •Electrical safety
- •Power tool use and care
- •Service
- •Рабочее место
- •Поддерживайте чистоту и порядок на Вашем рабочем месте.
- •Меры безопасности при подключении к электросети.
- •Указания по безопасности
- •Использование инструмента и уход за ним.
- •Important safety instructions
- •Translate into English:
- •Устройство и принцип работы р ис. 1. Внешний вид аппарата.
- •6 Claims, 5 Drawing Figures exhaust gas recirculation apparatus for engine with turbocharger
- •Read the patent given above, identify its parts and be ready to comment on peculiarities of their translation. 2. Translate the following extract into English:
- •Beltline: The horizontal area of the body along the door just below the side-window glass.
- •Abstract
- •Description
Translate into English:
Способность углерода соединяться с большинством элементов и образовывать молекулы самого разного состава и строения обусловливает огромное многообразие органических соединений. Эти реакции происходят под воздействием химических связей.
Химическая связь - взаимодействие атомов, обусловливающее их соединение в молекулы и кристаллы. Действующие при образовании химических связей силы
имеют в основном электрическую природу. Образование химических связей сопровождается перестройкой электронных уровней связывающихся атомов. Химические связи классифицируются по характеру распределения электронной плотности между атомами, образующими связь, в частности по симметрии распределения и другим признакам. К основным типам химических связей относятся такие как ковалентные, координационные, ионные, водородные и металлические.
Translate the following text into Russian:
“Town gas” is the name given to the gas with which many Americans cook. Town gas that is produced by the gasification of solid fuels contains - after removal of tar, ammonia and benzene - about 50% hydrogen, 20 to 30% methane and 6 to 17% carbon monoxide( percentages by volume) In addition, the gas contains some carbon dioxide, nitrogen and other impurities. Detoxication consists in removing the carbon monoxide, a highly poisonous colorless and odorless gas. It does not sustain combustion, but bums with a characteristic blue flame.
In order to obtain a nontoxic gas, it is necessary to reduce the carbon monoxide content to between 1 and 1,5%. This can be fairly easy achieved by conversation of carbon monoxide by means of water vapor(steam):
CO + HIO-+ COI + HI
In this process, stream and carbon monoxide, at a temperature of400° - 480° С and atmospheric pressure, are passed over a catalyst. The town gas flows upwards through a spray tower in which hot water is admitted at the top. As a result, the gas becomes saturated with water vapor.
Translate the following information into English:
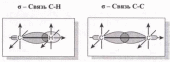
Химическое строение (порядок соединения атомов в молекулах) простейших алканов - метана, этана и пропана - показывают их структурные формулы. Из этих формул видно, что в алканах имеются два типа химических связей:
С-С и С-Н.
Связь С-С является ковалентной неполярной. Связь С-Н - ковалентная слабополярная, т.к. углерод и водород близки по электроотрицательности (2.5 - для углерода и 2.1 - для водорода). Образование ковалентных связей в алканах за счет общих электронных пар атомов углерода и водорода можно показать с помощью электронных формул:

Электронные и структурные формулы отражают химическое строение, но не дают представления о пространственном строении молекул, которое существенно влияет на свойства вещества.
Пространственное строение, т.е. взаимное расположение атомов молекулы в пространстве, зависит от направленности атомных орбиталей (АО) этих атомов. В углеводородах главную роль играет пространственная ориентация атомных орбиталей углерода, поскольку сферическая 1 s-AO атома водорода лишена определенной направленности.
Пространственное расположение АО углерода в свою очередь зависит от типа его гибридизации. Насыщенный атом углерода в алканах связан с четырьмя другими атомами. Следовательно, его состояние соответствует зр3-гибридизации. В этом случае каждая из четырех sp3-гибридных АО углерода участвует в осевом (о-) перекрывании с s-AO водорода или с sp3-AO другого атома углерода, образуя a-связи С-Н или С-С.
Четыре ст-связи углерода направлены в пространстве под углом 109°28’, что соответствует наименьшему отталкиванию электронов. Поэтому молекула простейшего представителя алканов - метана СН4 - имеет форму тетраэдра, в центре которого находится атом углерода, а в вершинах - атомы водорода:
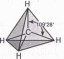
Валентный угол Н-С-Н равен 109°28\ Пространственное строение метана можно показать с помощью объемных (масштабных) и шаростержневых моделей.
L
CHAPTER VI REFINERY
esson # 1WHAT IS A REFINERY?
A refinery is a factory. Just as a paper mill turns lumber into legal
pads or a glassworks turns silica into stemware, a refinery takes a
raw material-crude oil—and transforms it into gasoline and
hundreds of other useful products. Inside a maze of silver
towers and pipes is a fascinating factory that changes hydrocarbon
molecules to make gasoline.
refinery is a factory. Just as a paper mill turns lumber into legal
pads or a glassworks turns silica into stemware, a refinery takes a
raw material-crude oil—and transforms it into gasoline and
hundreds of other useful products. Inside a maze of silver
towers and pipes is a fascinating factory that changes hydrocarbon
molecules to make gasoline.
A typical large refinery costs billions of dollars to build and millions more to maintain and upgrade. It runs around the clock 365 days a year, employs between 1,000 and
people and occupies as much land as several hundred football fields. It’s so big and sprawling, in fact, that workers ride bicycles from one station to another.
Chevron has five gasoline-producing “Factories” in the United States and another in Burnaby, British Columbia. Chevron Texaco has refining capacities worldwide of over two million barrels per day.
These world class operations had surprisingly humble origins. In 1876, company pioneers used wagons and mules to haul two primitive stills to a spot near Pico Canyon, Calif., the site of California’s first producing oil wells. The stills, each about the size of a garage, were used to heat oil at the prodigious rate of 25 to 40 barrels a day. This “oil boiling” produced kerosene, lubricants, waxes and gasoline—a clear, lightweight, liquid that generally was discarded as a useless byproduct. Gasoline’s lowly status rose quickly after 1892, when Charles Duryea built the first U.S. gas-powered automobile. From then on, the light stuff from crude oil became the right stuff.
Today, some refineries can turn more than half of every 42-gallon barrel of crude oil into gasoline. That’s a remarkable technological improvement from 70 years ago, when only 11 gallons of gasoline could be produced. How does this transformation take place? Essentially, refining breaks crude oil down into its various components, which then are selectively reconfigured into new products.
This process takes place inside a maze of hardware that one observer has likened to “a metal spaghetti factory.” Employees regulate refinery operations from within highly automated control rooms. Because so much activity happens out of sight, refineries are surprisingly quiet places. The only sound most visitors hear is the constant, low hum of heavy equipment.
The complexity of this equipment varies from one refinery to the next. In general, the more sophisticated a refinery, the better its ability to upgrade crude oil into high- value products. Whether simple or complex, however, all refineries perform three basic steps: separation, conversion and treatment.
Separation: heavy on the bottom, light on the top
Modem separation - which is not terribly different from the “cooking” methods used at the Pico Canyon stills - involves piping oil through hot furnaces. The resulting liquids and vapors are discharged into distillation towers, the tall, narrow columns that give refineries their distinctive skylines.
Inside the towers, the liquids and vapors separate into components or fractions according to weight and boiling point. The lightest fractions, including gasoline and liquid petroleum gas (LPG), vaporize and rise to the top of the tower, where they condense back to liquids. Medium weight liquids, including kerosene and diesel oil distillates, stay in the middle. Heavier liquids, called gas oils, separate lower down, while the heaviest fractions with the highest boiling points settle at the bottom. These tarlike fractions, called residuum, are literally the “bottom of the barrel.”
The fractions now are ready for piping to the next station or plant within the refinery. Some components require relatively little additional processing to become asphalt base or jet fuel. However, most molecules that are destined to become high-value products require much more processing.
Conversion: cracking and rearranging molecules to add value
This is where refining fanciest footwork takes place—where fractions from the distillation towers are transformed into streams (intermediate components) that eventually become finished products. This also is where a refinery makes money, because only through conversion can most low-value fractions become gasoline.
The most widely used conversion method is called cracking because it uses heat and pressure to “crack” heavy hydrocarbon molecules into lighter ones. A cracking unit consists of one or more tall, thick-walled, bullet-shaped reactors and a network of furnaces, heat exchangers and other vessels.
Fluid catalytic cracking, or “cat cracking,” is the basic gasoline-making process. Using intense heat (about 1,000 degrees Fahrenheit), low pressure and a powdered catalyst (a substance that accelerates chemical reactions), the cat cracker can convert most relatively heavy fractions into smaller gasoline molecules.
Hydrocracking applies the same principles but uses a different catalyst, slightly lower temperatures, much greater pressure and hydrogen to obtain chemical reactions. Although not all refineries employ hydrocracking, Chevron is an industry leader in using this technology to cost-effectively convert medium- to heavyweight gas oils into high-value streams. The company’s patented hydrocracking process, which takes place in the Isocracker unit, produces mostly gasoline and jet fuel.
Some Chevron refineries also have cokers, which use heat and moderate pressure to turn residuum into lighter products and a hard, coallike substance that is used as an industrial fuel. Cokers are among the more peculiar-looking refinery structures. They resemble a series of giant drums with metal derricks on top.
Cracking and coking are not the only forms of conversion. Other refinery processes, instead of splitting molecules, rearrange them to add value. Alkylation, for example, makes gasoline components by combining some of the gaseous byproducts of cracking. The process, which essentially is cracking in reverse, takes place in a series of large, horizontal vessels and tall, skinny towers that loom above other refinery structures.
Reforming uses heat, moderate pressure and catalysts to turn naphtha, a light, relatively low-value fraction, into high-octane gasoline components. Chevron’s patented reforming process is called Rheniforming for the rheniumplatinum catalyst used.
Treatment: the finishing touch
Back when Chevron’s founders boiled crude oil to get kerosene, they didn’t have to worry about customer specifications or government standards. Today, however, a major portion of refining involves blending, purifying, fine-tuning and otherwise improving products to meet these requirements.
To make Chevron gasoline, refinery technicians carefully combine a variety of streams from the processing units. Among the variables that determine the blend are octane level, vapor pressure ratings and special considerations, such as whether the gasoline will be used at high altitudes. Technicians also add Techron, Chevron’s patented performance additive, and dyes that distinguish the various grades of fuel.
Refining has come a long way since the oil boiling days of Pico Canyon. By the time a gallon of gasoline is pumped into a car’s tank, it contains more than 200 hydrocarbons and additives. All that changing of molecules pays off in a product that ensures smooth, high-performance driving.
WORDLIST
Oil refinery Нефтеперегонный завод
crude oil, petroleum сырая нефть
glassworks стеклянная посуда, изделия
stemware столовое стекло
still перегонный куб, дистиллятор
separation сепарация; отделение, очистка
conversion переработка (конверсия газов)
treatment технологическая обработка;
очистка
distillation tower ректификационная или
дистилляционная колонна
liquid petroleum gas сжиженный нефтяной газ
(LPG)gas oil газовое масло, газойль
distillate дистиллят, нефтепродукт, отстой;
residuum тяжелые остатки прямой перегонки
plant (зд.) установка; завод
stream (зд)промежуточный нефтепродукт
fluid catalytic cracking жидко-каталитический крекинг
cat cracker установка каталитического
крекинга
reforming реформинг (т.е. повторная
переработка)
coker установка для коксования,
коксовик
derrick подъёмник (вышка)
naphtha лигроин(керосин)
blending смешивание (с добавлением
присадок)
additive присадка
fine-tuning доведение до...; доводка
jet fuel реактивное топливо
alkylation алкилирование; алкикатализатор
gas bag газовая пробка (мешок)
hydrogen chloride хлорид водорода(соляная кислота)
hydrogen sulfide сероводород
naphthenic acids нафтеновые кислоты
demulsifies антиэмульгаторы
diatomaceous earth инфузорная (диатомитовая) земля
GLOSSORY:
Antiknock compound. A chemical added to gasoline to reduce its tendency to knock. This additive makes it more difficult to ignite the gasoline and air mixture and thus raises its octane number.
Cracking. The separation of a large hydrocarbon molecule into two or more smaller hydrocarbon molecules.
Reforming. Removal of hydrogen atoms from a cycloparaffin to create an aromatichydrocarbon.
Octane number. A measure of a gasoline’s resistance to knocking. The higher the octane number, the harder it is to ignite the fuel and air mixture and the less likely it is to knock.
Residual liquid. The liquid that falls to the bottom of a distillation tower because it contains molecules that rarely become gaseous at the temperatures of the tower.
Volatile. A material that easily becomes gaseous because its molecules are not strongly attached to one another.
Separator (P-tank, surge tank) a device in the form of a tank designed for separation of oil from gas and water. Separators may be vertical or horizontal, two-phase or three phase. Some of them are also capable of separating oil from solids due to special filters. The gravity and centrifugal forces are used for the separation process.
EXERCISES
