
- •Internal combustion engine
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Using the English-Russian dictionary translate the following text
- •Into Russian:
- •IV. Translate the following information into English:
- •Translate the following sentences into Russian:
- •Explain and translate the following definitions of the car body elements:
- •Translate the following information into English:
- •Translate the following information into Russian:
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Translate the following information into English:
- •Translate the following text into Russian:
- •Give equivalents to the following words and word combinations:
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate the following text into Russian:
- •Be ready to talk and discuss the general structure of a ship
- •Uss Nimitz’s catapult 1
- •Translate the following text into English:
- •Inspecting a used car
- •Incandescent lighting - освещение лампами накаливания
- •Answer and translate the following questions:
- •Translate the following sentences:
- •Translate the following text into Russian:
- •IV. Translate the following information into English:
- •Various definitions
- •Decipher and translate the following abbreviations:
- •Answer the following question:
- •Translate the following text into Russian:
- •Translate the following material into English:
- •I. Translate the following sentences:
- •II. Translate the following information in to Russian:
- •Translate the following advertising material into English:
- •Be ready to talk about the power supply at your home.
- •Introduction to power electronics
- •I. Answer the following questions:
- •Translate into English words in brackets and then the sentences
- •Into Russian:
- •Translate the following text: Conductors
- •Translate the following sentences:
- •Translate into English: Теория цепей
- •Translate into Russian: Current supply
- •Give equivalents to the following words and word combinations:
- •Translate and answer the following questions:
- •Translate the following text:
- •Translate the text and be ready to discuss the general radio design:
- •Translate into English the following material:
- •Translate the following extract: Microphone transmitter.
- •Introduction to radar fundamentals
- •Give equivalents to the following words and word combinations:
- •Put 10 questions to the text and answer them:
- •Translate into Russian the following text:
- •Translate into English:
- •Read and translate the list of chemical elements with their symbols and atomic number: (in alphabetical order)
- •Translate the following sentences:
- •Translate into Russian:
- •Translate into English:
- •Translate the following information into English:
- •Put 6 questions to the text and answer them:
- •Translate the following sentences in to Russian:
- •Translate the following text and he ready to discuses properties of elements: Chemical properties of elements
- •Vanderwaals radius
- •Ionic radius
- •Isotopes
- •Translate the following text into English using proper terms given below:
- •Classification by Structural Change
- •Classification by Reaction Type
- •Translate into Russian the following sentences:
- •Translate the follow text into Russian:
- •Translate the classification of hydrocarbons into Russian:
- •Reaction Characteristics
- •Factors that Influence Reactions
- •Translate the following information into Russian:
- •Translate the following text into English: Насыщенные углеводороды
- •Translate the following material into Russian:
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate into English:
- •Translate the following text into Russian:
- •Translate the following information into English:
- •Translate the words and word combinations in brackets into English.
- •Give equivalents to the following word combinations:
- •Translate the following text into Russian:
- •Translate into English:
- •Translate into Russian:
- •Be ready to answer questions on the text and talk about the refinery presses.
- •Translate words and word combinations in brackets into English. Translate
- •Give equivalents to the following word combinations:
- •Translate the following text:
- •Translate into English:
- •Translate into Russian:
- •Translate into English:
- •Translate in to English:
- •Interface поверхность раздела; граница
- •Viscosity
- •Translate words and word combinations in brackets into English. Then
- •Translate the following text and be ready to discuss it: crude oil pretreatment (desalting).
- •Electrostatic desalting.
- •Translate the following sentences into Russian:
- •Translate the following information into Russian:
- •Translate the following text into English:
- •Translate the following information into English:
- •Give eqvivalents to the following words and word combinations:
- •Answer the following questions:
- •Translate the following sentences:
- •Translate the following text into English:
- •Translate the following text:
- •Translate into English:
- •Improving the quality of petroleum products
- •Give eqvivalents to the following words and word combinations:
- •Answer the following questions:
- •Translate into Russian:
- •Translate into English:
- •Translate into Russian: The Physics of Oil Refineries
- •Discussion of the refinery process. Prepare the brief report on one of the theme topics.
- •Translate the following sentences:
- •Translate the following text in writing:
- •Introduction to nuclear power
- •Translate the following sentences into Russian:
- •Translate the following text into Russian:
- •Translate the following text into English:
- •Inside the reactor
- •Put some questions to the text and translate them:
- •Translate the following sentences:
- •Translate the following material into English:
- •Translate the following text into Russian:
- •Translate the following text into Russian:
- •Translate the following word combination into Russian:
- •Translate the following sentences into Russian:
- •Translate the following text into English:
- •Translate the following text into English:
- •Translate the following extract into English:
- •Translate the following sentences:
- •Translate the following information into Russian:
- •Translate into Russian:
- •Translate the following information into English:
- •Translate the following information into English:
- •Give equivalents to the terms on the fig.2 using technical dictionaries.
- •Translate into Russian:
- •Translate into Russian:
- •Translate the following information into Russian:
- •Translate into English: Устройство энергетических ядерных реакторов.
- •Put several questions to the text above and answer them:
- •Translate the following text into Russian:
- •Translate the following extract into Russian:
- •To check yourself try to translate the following text at sight:
- •Introduction to the almr/prism
- •Translate the following information into English: хранение ядерного топлива
- •Work area
- •Electrical safety
- •Power tool use and care
- •Service
- •Рабочее место
- •Поддерживайте чистоту и порядок на Вашем рабочем месте.
- •Меры безопасности при подключении к электросети.
- •Указания по безопасности
- •Использование инструмента и уход за ним.
- •Important safety instructions
- •Translate into English:
- •Устройство и принцип работы р ис. 1. Внешний вид аппарата.
- •6 Claims, 5 Drawing Figures exhaust gas recirculation apparatus for engine with turbocharger
- •Read the patent given above, identify its parts and be ready to comment on peculiarities of their translation. 2. Translate the following extract into English:
- •Beltline: The horizontal area of the body along the door just below the side-window glass.
- •Abstract
- •Description
Translate into Russian the following sentences:
The reactant is often, but not always the larger and more complex molecule in the chemical reactions.
Catalysts are substances that accelerate the velocity of a chemical reaction without themselves being consumed or appearing as part of the reaction product.
The best way to achieve proficiency in organic chemistry is to understand how reactions take place, and to recognize the various factors that influence their course.
Substitution reactions, as the name implies, are characterized by replacement of an atom or group (Y) by another atom or group (Z).
These reactions are general for most functional groups, including those incorporating carbon-oxygen double bonds and carbon-nitrogen double and triple bonds.
Many organic reactions are catalyzed by acids and/or bases, and although such transformations may seem complex, our understanding of how they occur often begins with the functioning of the catalyst.
The relative strength of a group of acids may be evaluated by measuring the extent of reaction that each group member undergoes with a common acid.
Many organic reactions take place in aqueous environments (living cells), or worked-up in water, it is important to consider how a conjugate acid-base equilibrium mixture changes with pH.
If the carbon chlorine bond in this complex breaks with both the bonding electrons remaining with the more electronegative atom (chlorine), the carbon assumes a positive charge.
Translate the follow text into Russian:
Ammonia (NH|) is a colorless gas which can readily be liquefied by compression. The liquid is colorless and highly refractive and has a boiling point of— 33 Ц C. Because of its high heat of vaporization, ammonia is widely used in refrigeration. Most ammonia is produced on a large scale by special chemical^plants. In this process, hydrogen and nitrogen are combined at a temperature of 500fcC and pressure of 200 atm. according to the reaction 3 HI + N1 —► 2 NH£ which takes place in contact with a catalyst consisting of iron stabilized with aluminum oxide and potassium oxide. The initial materials are obtained from air and water, the oxygen being removed by means of carbon( in the form of coke) as a reducing agent. At elevated temperature, air is converted in a gas producer according to the following reaction:
4NI + OI + 2C—>4NI + 2 CO
whereby so-called producer gas is formed. As a result of this reaction, in which air is blown through the gas producer, the coke charge in the latter is heated to incandescence. Then water vapor (steam) is passed through the incandescent coke and is decomposed into co-called water gas, a mixture of hydrogen and carbon monoxide, as follows:
С + HIO —> HI + CO
As a result of passing stream through the coke, the temperature in the gas producer is lowered. The cycle can then be repeated by blowing air again, to yield producer gas and heat up the coke, and so on.
Translate the classification of hydrocarbons into Russian:
Классификацию углеводородов проводят по следующим структурным признакам, определяющим свойства этих соединений:
строение углеродной цепи (углеродного скелета);
наличие в цепи кратных связей С=С и С=С (степень насыщенности).
В зависимости от строения углеродной цепи углеводороды подразделяют на две группы:
ациклические или алифатические, т.е. “жирные” (от греческого слова “алейфар” - “жир”, т.к. впервые структуры с длинными углеродными цепями были обнаружены в составе жиров);
циклические.
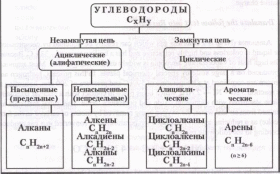
Открытая (незамкнутая) цепь алифатических углеводородов может быть неразветвленной или разветвленной. Углеводороды с неразветвленной углеродной цепью называют нормальными (н-) углеводородами. Среди циклических углеводородов выделяют:
алициклические (т.е. алифатические циклические);
ароматические (арены).
В этом случае классификационным признаком служит строение цикла.
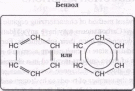
К ароматическим углеводородам относят соединения, содержащие один или несколько бензольных циклов (формула бензола С6Н6).
По степени насыщенности различают:
насыщенные (предельные) углеводороды (алканы и циклоалканы), в которых имеются только простые связи С-С и отсутствуют кратные связи;
ненасыщенные (непредельные), содержащие наряду с одинарными связями С-С двойные и/или тройные связи (алкены, алкадиены, алкины, циклоалкены, циклоалкины).
Следует заметить, что хотя по составу бензол С6Н6 формально соответствует ненасыщенным циклическим углеводородам (его молекулу часто изображают как шестичленный цикл с тремя двойными связями), то соединения ряда бензола относят к самостоятельной группе ароматических углеводородов (аренов).
LESSON # 4
OXIDATION AND REDUCTION REACTIONS
A parallel and independent method of characterizing organic reactions is by oxida- tion-reduction terminology. Carbon atoms may have any oxidation state from -4 (e.g. CH4 ) to +4 (e.g. C02 ), depending upon their substituents. Fortunately, we need not determine the absolute oxidation state of each carbon atom in a molecule, but only the change in oxidation state of those carbons involved in a chemical transformation. To determine whether a carbon atom has undergone a redox change during a reaction we simply note any changes in the number of bonds to hydrogen and the number of bonds to more electronegative atoms such as O, N, F, Cl, Br, I, & S that has occurred. Bonds to other carbon atoms are ignored. This count should be conducted for each carbon atom undergoing any change during a reaction.
If the number of hydrogen atoms bonded to a carbon increases, and/or if the number of bonds to more electronegative atoms decreases, the carbon in question has been reduced (i.e. it is in a lower oxidation state).
If the number of hydrogen atoms bonded to a carbon decreases, and/or if the number of bonds to more electronegative atoms increases, the carbon in question has been oxidized (i.e. it is in a higher oxidation state).
If there has been no change in the number of such bonds, then the carbon in question has not changed its oxidation state. In the hydrolysis reaction of a nitrile, the carbon has not changed its oxidation state.
These rules are illustrated by the following four addition reactions involving the same starting material, cyclohexene. Carbon atoms colored blue are reduced, and those colored red are oxidized. In the addition of hydrogen both carbon atoms are reduced, and the overall reaction is termed a reduction. Peracid epoxidation and addition of bromine oxidize both carbon atoms, so these are termed oxidation reactions. Addition of HBr reduces one of the double bond carbon atoms and oxidizes the other; consequently, there is no overall redox change in the substrate molecule.
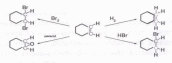
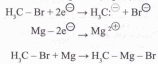
Since metals such as lithium and magnesium are less electronegative than hydrogen, their covalent bonds to carbon are polarized so that the carbon is negative (reduced) and the metal is positive (oxidized). In the following equation and half-reactions the carbon atom (blue) is reduced and the magnesium (magenta) is oxidized.
Classification by Functional Group
Functional groups are atoms or small groups of atoms (usually two to four) that exhibit a characteristic reactivity when treated with certain reagents. A particular functional group will almost always display its characteristic chemical behavior when it is present in a compound. Because of this, the discussion of organic reactions is often organized according to functional groups. The following table summarizes the general chemical behavior of the common functional groups.
Functional Class |
Formula |
Characteristic Reactions |
Alkanes |
C-C, C-H |
Substitution (ofH, commonly by Cl or Br) Combustion (conversion to C02 & H20) |
Alkenes |
C=C-C-H |
Addition Substitution (of H) |
Alkynes |
C=C-H |
Addition Substitution (of H) |
Alkyl Halides |
H-C-C-X |
Substitution (of X) Elimination (of HX) |
Alcohols |
H-C-C-O-H |
Substitution (of H); Substitution (of OH) Elimination (of HOH); Oxidation (elimination of 2H) |
Ethers |
(a)C-O-R |
Substitution (of OR); Substitution (of a-H) |
Amines |
C-NRH |
Substitution (of H); Addition (to N); Oxidation (of N) |
Benzene Ring |
C6H6 |
Substitution (of H) |
Aldehydes |
(a)C-CH=0 |
Addition Substitution (of H or a-H) |
Ketones |
(a)C-CR=0 |
Addition Substitution (of a-H) |
Carboxylic Acids |
(a)C-C02H |
Substitution (of H); Substitution (of OH) Substitution (of a-H); Addition (to C=0) |
Carboxylic Derivatives |
( a ) С - С Z = 0 (Z = OR, Cl, NHR, etc.) |
Substitution (ofZ); Substitution (of a-H) Addition (to C=0) |
This table does not include any reference to rearrangement, due to the fact that such reactions are found in all functional classes, and are highly dependent on the structure of the reactant. Furthermore, a review of the overall reaction patterns presented in this table discloses only a broad and rather non-specific set of reactivity trends. This is not surprising, since the three remaining categories provide only a coarse discrimination (comparable to identifying an object as animal, vegetable or mineral). Consequently, apparent similarities may fail to reflect important differences. For example, addition reactions to C=C are significantly different from additions to C=0, and substitution reactions of C-X proceed in very different ways, depending on the hybridization state of carbon.
