
- •Acetyl ch3 group is substituted with at least one phenyl ring for antimuscarinic activity
- •Identify which chemical class the following compounds belong to ..
- •Antimuscarinic drugs used in the treatment of urinary incontinence
- •Ipratropium hydrobromide
- •Ipratropium Hydrobromide (Atrovent®)
- •Resource: Rang and Dales Pharmacology, 6th Edition, Chapter 10, Fig. 10.2
- •Incorrect structure: 1935
- •10 Carbon bridge between two nitrogens
- •Vecuronium at the nicotinic receptor
- •17 Deacetylated metabolite is active
Events
and sites of drug action at a
nicotinic cholinergic synapse
Neuromuscular
Blockers
Postsynaptic
nicotinic
K+ ACh
receptor
Copyright
© 2D07 by Churchill Livingstone, an imprint of Elsevier Inc.
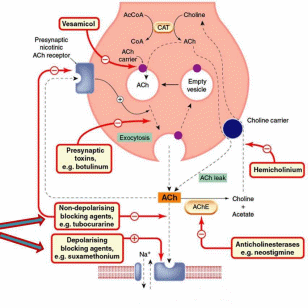
Resource: Rang and Dales Pharmacology, 6th Edition, Chapter 10, Fig. 10.2

Neuromuscular
Blockers (NMBs)
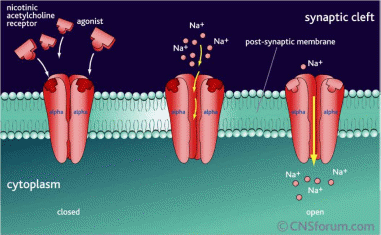
Depolarizing
blockers: Depolarizes
the post synaptic membrane with the inflow of sodium ions
Non-depolarizing
blockers: Blocks
the sodium ions conductance
Therapeutic
application
•
Neuromuscular
blocking agents are used
primarily as an adjunct
to general anesthesia.
◦ Produces
skeletal muscle relaxation which
facilitates operative
procedures
•
Questions
to be considered in choosing NMB
Will the compound produce
the desired
neuromuscular blockade?
What is its duration of
action?
What are its adverse
effects?
What is its relative cost?
Discovery:Tubocurarine
- the first neuromuscular blocker
The neuromuscular
blocking
effects of extracts
of curare were first
reported as early
as 1510
It was demonstrated
that
curare extracts prevented
skeletal
muscle
contractions by an effect at
the neuromuscular
junction
©
Cl


Tubocurarine
structure
CH3
©
N" Cl
V°H3
H3CO.
HO'
quaternary
ammonium
tertiary
amine
Cl
H^
H3C
CH3
N^ Cl
CH3
O
OCH3
Correct
Structure: 1970
Nevertheless, the incorrect
structure of tubocurarine served as the model for the synthesis of
all the neuromuscular blocking agents in use today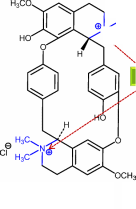


Incorrect structure: 1935
Rationale
for designing new И compounds
Bis-quaternary ammonium
compound having two quaternary ammonium salts separated by 10
to 12 carbon atoms (similar
to the distance between the nitrogen atoms in tubocurarine) was a
requirement for neuromuscular blocking activity
The rationale for this
structural requirement was that nicotinic receptors possessed
two anionic-binding sites,
both of which had to be occupied for a neuromuscular blocking
effect.
Nicotinic antagonist may be
a
Depolarizing
neuromuscular blocker
Non-depolarizing
neuromuscular blocker


Depolarizing
agents
•
Decamethonium
Bromide
Succinylcholine
Chloride (Anectine®)
Br
© CH,
©.
H3C—N—(CH2)io-
CH3
CH3
©
l© Br
N—CH3
CH,
Decamethonium
Bromide
Succinylcholine
Chloride

Decamethonium
Bromide
Br >1 CH3
©
НзС^Г(СН2)'°^СН3
сн\
/Jh,
8/s-quaternary ammonium salt
Decamethonium at the
nicotinic receptor

10 Carbon bridge between two nitrogens

Acetylcholine
at the nicotinic
receptor
site
Anionic binding pocket


Succinyl
choline
•••••
о
Dimer of acetylcholine bonded
through their a-carbon adjacent to ester
Rapidly hydrolyzed to
inactive metabolites, both in aqueous solution and plasma esterases
(shorter duration of action, 6-8 min)
Through succinylcholine has
10 atoms between two nitrogens, it still reported to catalyze with
two molecules at the receptor site.

Non-depolarizing
agents
Aminosteroids
Tetrahydroisoquinolines
Pancuronium
bromide
(Pavulon)
Vecuronium
bromide
(Norcuron)
Rocuronium bromide
(Zemuron)
Pipecuronium
bromide
(Arduan)
❖d-Tubocurarine
and metocurine (Metubine Iodide)
❖Atracurium
besylate (Tracrium)
❖Cis-atracurium
Mivacurium chloride
(Mivacron)
Doxacurium chloride
(Nuromax)

|
9 |
A 3 |
B |
V.
6
Monoquaternary
aminosteroids are less potent but has faster onset of action
