
- •1) Carbohydrates. Concept.Biological role.Classification.
- •2) Structureofmonosaccharide, classification.
- •Structure and nomenclature
- •Linear-chain monosaccharides
- •3) Isomerismofmonosaccharide (stereoisomerism, cyclo-, oxo-tautomerism, conformation).
- •4) Chemical properties of monosaccharide.
- •5) Qualitative reactions on monosaccharide
- •6) Derivativesof monosaccharide: deoxysacarose, aminosacarose, neiramine acid, ascorbic acid.
Topic№13: Carbohydrates. Monosaccharide.
Basic questions:
1.Carbohydrates.Concept.Classification.
2. Structureofmonosaccharide, classification.
a) carbonyl formula of Fisher
b) half-acetylic formula of Colly-Tollence
c) cyclic formula of Cheourse
3. Isomerismofmonosaccharide (stereoisomerism, cyclo-, oxo-tautomerism, conformation).
4. Chemical properties of monosaccharide.
а) oxidation of monosaccharide (gluconic, glucaric, glucoronic acids)
b) reduction of monosaccharide
c) formation of О- and N-glycosides
5. Qualitative reactions on monosaccharide
6. Derivativesof monosaccharide: deoxysacarose, aminosacarose, neiramine acid, ascorbic acid.
1) Carbohydrates. Concept.Biological role.Classification.
A carbohydrate is an organic compound that consists only of carbon, hydrogen, and oxygen, usually with a hydrogen: oxygen atom ratio of 2:1; in other words, with the empirical formula Cm(H2O)n. Carbohydrates are solid substances, dissolved in water and undissolved in alcohols and etheos. Always solutions of carbohydrates gives neutral reaction of the lacmus.
w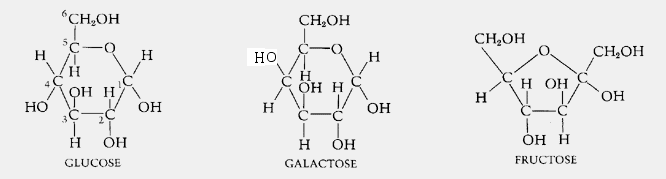 ith
a hydrogen:oxygen atomratio
of 2:1; in other words, with the empirical formula Cm(H2O)n.
ith
a hydrogen:oxygen atomratio
of 2:1; in other words, with the empirical formula Cm(H2O)n.
Biological role:
Energetic. If oxidation one gram of carbohydrates makes free 4.2 ecol of energy.
Carbohydrates are plastic material for the formation of complex proteins, nucklic acids, lipids.
Protective function is carried by mucopolysaccharides
Heparin (complex carbohydrates) is the natural anticoagulant and take off coagulation of blood.
Carbohydrates and its derivatives have found an application as medicine like glucose, saccarose, calcium gluconate, glycosides.
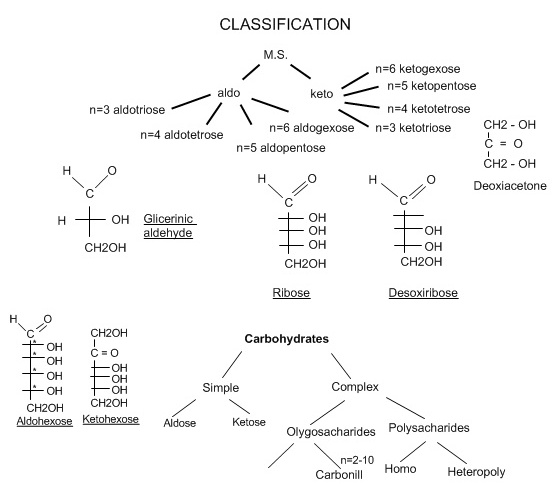
2) Structureofmonosaccharide, classification.
a) carbonyl formula of Fisher
b) half-acetylic formula of Colly-Tollence
c) cyclic formula of Cheourse
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste.
Examples of monosaccharides include glucose (dextrose), fructose (levulose),galactose, xylose and ribose. Monosaccharides are the building blocks of disaccharides such as sucrose and polysaccharides (such as cellulose and starch).
CommonCarbohydrates |
|
Name |
Derivation of name and Source |
Monosaccharides |
|
Glucose C6H12O6 |
From Greek word for sweet wine; grape sugar, blood sugar, dextrose. |
Galactose C6H12O6 |
Greek word for milk--"galact", found as a component of lactose in milk. |
Fructose C6H12O6 |
Latin word for fruit--"fructus", also known as levulose, found in fruits and honey; sweetest sugar. |
Ribose(C5H10O5) |
Ribose and Deoxyribose are found in the backbone structure of RNA and DNA, respectively. |
Number of Carbons: Monosaccharides can be further classified by the number of carbons present. Hexoses (6-carbons) are by far the most prevalent.
|
|||||||||||||||
Functional Groups: Aldoses contain the aldehyde group - Monosaccharides in this group are glucose, galactose, ribose, and glyceraldehyde. Ketoses contain the ketone group - The major sugar in this group is fructose. Reducing: Contain a hemiacetal or hemiketal group. Sugars include, glucose, galactose, fructose, maltose, lactose Non-reducing: Contain no hemiacetal groups. Sucrose and all polysaccharides are in this group. |
|||||||||||||||
