
Graph 1
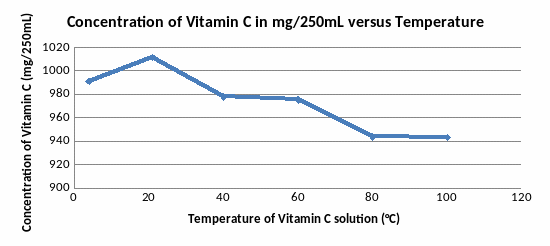 By
observing the insert line graph of all results above, the highest
point can be identified: 21°C (room temperature) with concentration
of Vitamin C is 1011.6mg/250mL. The graph is increased before this
point and decreased after. It makes me think that the temperature
highest and lower than 21°C destroys Vitamin C. However, my graph
has only one point is lower than 21°C, so it is hard to conclude
that the cold destroys Vitamin C.
By
observing the insert line graph of all results above, the highest
point can be identified: 21°C (room temperature) with concentration
of Vitamin C is 1011.6mg/250mL. The graph is increased before this
point and decreased after. It makes me think that the temperature
highest and lower than 21°C destroys Vitamin C. However, my graph
has only one point is lower than 21°C, so it is hard to conclude
that the cold destroys Vitamin C.
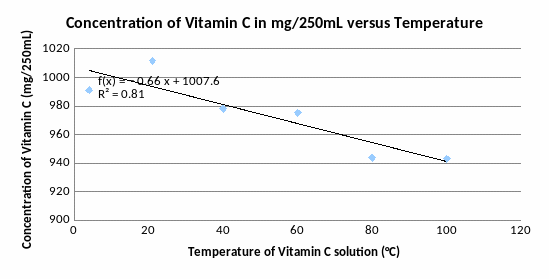
By observing the graph above, two outliers within the data can be identified: 4°C (too low concentration of Vitamin C for this temperature) and 100 °C (too high concentration of Vitamin C for this temperature). If you discount these outliers, a more believable model can be observed:
Graph 2
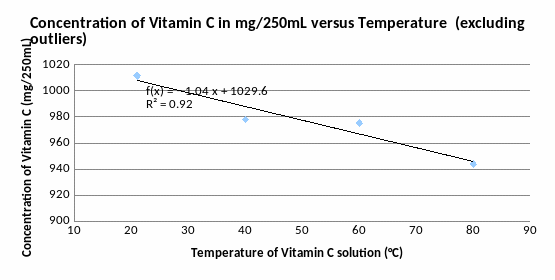
This graph shows a more agreeable and straight model. The R² value’s 0,9183 makes this a much more reliable model than previous. Reasons for the two outliers can be found in discussion.
Conclusion:
The aim of investigation was to find out the effect of temperature on concentration of Vitamin C (C6H8O6). The model of the temperature versus concentration Vitamin C in solution above displays a strong negative relationship, which meant that the concentration of Vitamin C declined over the temperature. The Vitamin C concentration started off being 1011.6 mg/250mL, and declined by 1,043 mg/250mL every 20 °C on average (see graph 2), until it reached 943.8 mg/250mL when temperature was 80°C. It proves my hypothesis that increasing of temperature will decrease the concentration of Vitamin C in solution, but as my data has only one point of temperature that lower than the room temperature as it is hard to prove or disprove second part of my hypothesis that decreasing of temperature will also decrease the concentration of Vitamin C in solution.
Discussion:
Significance of findings:
The conclusion of my investigation stated that amount of Vitamin C declined over the temperature. This could be a useful piece of information for cooks, because they might know that heat decreases Vitamin C content. Therefore, the investigation supports the idea that eating fresh fruits and vegetables is the best way to keep ones Vitamin C level up.
My investigation could help food corporations. They could know that it is bad to heat fruits and vegetable, which includes Vitamin C, because it makes them poor for vitamin C. Therefore, this food is less healthful for people. It means it is much beneficial for food corporations don’t heat food without necessary, making food more healthful.
Also my nutritionists and people, who think about their health, could use my investigation for making some diet for them.
Significance of the results in reaction to the background information and validity of conclusion:
The reason for the declination of concentration of Vitamin C is that the Vitamin C molecules get kinetic energy by heated, so there are more collisions between particles occur and so more molecules of Vitamin C reacts with other chemical molecules, such as water, in the solution and so the overall amount of Vitamin C decreases (from Law of chemical collisions). As it increasing number of collisions over the increasing temperature, there is smaller number of remained Vitamin C particles, which could reduce smaller number of I2 to iodine ions, and therefore more iodine were left in the solution to react with Na2S2O3 solution, which made the values in back titrations increase, and the difference decrease given that the blank value stayed constant. Thus, as temperature increased, more collisions of Vitamin C happened and the concentration of Vitamin C being stored decreased.
In the structure of ascorbic acid there is substantial strain in carbon rings that involve 4 or less carbon atoms (see the background information). This is called internal strain or I strain. As heat rises, the strain rises and ultimately the ring breaks due to the strain. But this breaking occurs by the temperature at 553°C and higherxii. I did not use so high temperatures in my practice, so this reason does not have effect for my data. It means my conclusion does not valid for temperature, which higher than 553°C.
If the solution with Vitamin C is exposed to a low temperature for a long time you will actually destroy the vitamin Cxiii. So my conclusion is not valid to low temperatures.
If others will try to repeat my investigation, the different storage conditions over the temperature could get effect on Vitamin C, so others could get different data for same topic, but the trend should show a common negative relationship. For example, the investigation of scientists’ ‘Temperature Effects on Vitamin C Content in Citrus Fruits’xiv. On other side, the difference in the initial Vitamin C concentration did not affect the results of the investigation much, because the trend would still be obtained for the effect of temperature on Vitamin C concentration, only that the values for Vitamin C concentration were lower/higher in parallel sense at each temperature.
Possible improvements:
As with any conclusion made from analytical analysis of data, reliability could by improved by increases the data sample size. With reference to our investigation, this could be of great benefit, as there were only 6 data sets used, and 2 turned out to be outliers. If more temperatures were taken, such as -20°C, -10C, 0°C, 20°C, 30C, 40°C, 50C, 60°C, 70C, 80°C, 100°C a stronger correlation between two variables could have been observed, or the relationship between them could be completely disproved.
My data would be more correct, if there will be more repeating for each time titrations.
It is better to do another investigation with stable conditions such as temperature and humidity.
Errors
It was hard to keep one exactly temperature about 10 minutes by using ice. So I have different in my temperature is about 1-2°C.
I measured the time by stopwatch, so it could have an error of human reaction. So to make the time, which is exactly 10 minutes for each flask having a state temperature, is impossible.
In the investigation, we used different temperatures. Before doing titrations, I had to wait before the temperature of solution cools or warms to room temperature, because only in room temperature I can do my titrations and be sure that my pipette or beaker would not expand. Therefore, there was different time for each solution become to room temperature, and it could have effect on my concentration of Vitamin C, because the oxidation of Vitamin C is occurredxv. I tried to minimize the oxidation by using foil, but still there is air in the conical flask, which reacts with Vitamin C. For example, my outliers of 4°C and 100°C have the biggest time (compared with other temperatures) before become to room temperatures. In my opinion, the time could be a reason of that they are unusual points on my graph.
Room temperature could have effect on my investigation, because it’s possibly was changing within the 7 hours’ practice.
iRecourse:
Relevant background information:
Vit C-
http://docsdrive.com/pdfs/ansinet/pjn/2011/1168-1169.pdf
ii http://schools-wikipedia.org/wp/v/Vitamin_C.htm
iii http://en.wikipedia.org/wiki/Ascorbic_acid
iv http://www.nlm.nih.gov/medlineplus/ency/article/002404.htm
v http://www.worldofmolecules.com/antioxidants/vitaminc.htm
