
Ann Agapova
Report for Investigation on Vitamin C concentration
Purpose
Aim
The aim of investigate is the effect of temperature (expressed in °C) on the concentration of Vitamin C (expressed in mg/250mL).
Independent variable is Temperature (°C). Possible range: -20°C, 0°C, 20°C, 40°C, 60°C, 80°C, 100°C.
Dependent variable is Concentration of Vitamin C (expressed in mg/250mL)
Hypothesis
I am going to predict that increasing and decreasing of temperature will decrease the concentration of Vitamin C in solution. I have read report ‘Temperature Effects on Vitamin C Content in Citrus Fruits’ by P.C. Njoku, A.A. Ayuk and C.V. Okoyei, which about the effect of temperature on the concentration of Vitamin C. Based on that, I can predict that my hypothesis is correct.
Relevant background information:
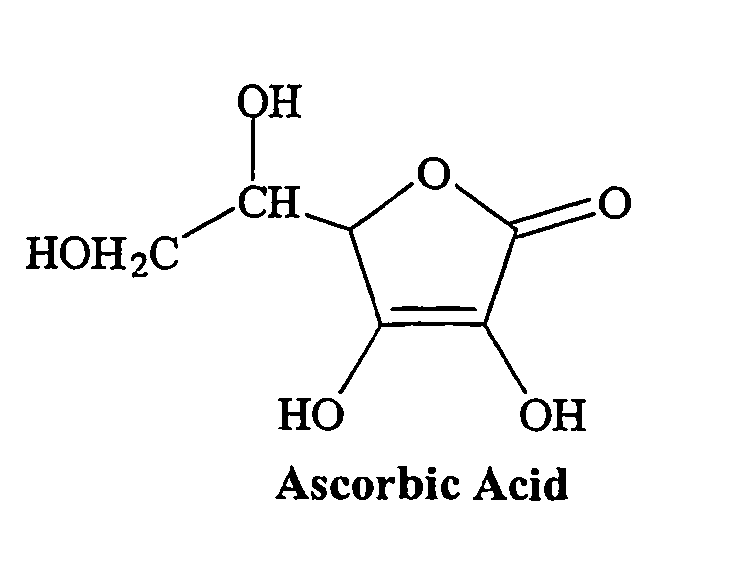
Vitamin C
Vitamin C is also known as ascorbic acid. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. The melting point of Vitamin C is 553°Cii. Vitamin C is needed for the growth and repair of tissues in all parts of your body. Vitamin C is one of many antioxidants. Antioxidants are nutrients that block some of the damage caused by free radicals. The body is not able to make vitamin C on its own, and it does not store vitamin C. It is therefore important to include plenty of vitamin C-containing foods in your daily diet. All fresh fruits and vegetables contain some amount of vitamin Ciii. The Recommended Dietary Allowance (RDA) for vitamins reflects how much of each vitamin most people should get each day. The RDA for vitamins may be used as goals for each person. The average amount of Vitamin C per day is 90 mg for men and 75 mg for womeniv.
Vitamin C has the chemical formula C6H8O6 and a molecular mass of 176.14 grams per molv.
Iodine
Iodine is a chemical element with symbol I. It has dark purple/ dark colour. Elemental iodine is slightly soluble in water. Iodine is found on Earth mainly as the highly water-soluble iodide ion (I−). Like the other halogens, free iodine occurs mainly as a diatomic molecule I2, and then only momentarily after being oxidized from iodide by an oxidant like free oxygenvi.
Iodine is useful to the body but an overdose is damaging, when intake is more than 1.1 mg/dayvii. Iodine is toxic to the skin and eyesviii, so it would be careful use in practice work. If a contact between iodine and skin or eyes is happened, the cold water can be used for washing it.
Potassium iodate
Potassium iodate (KIO3) is a chemical compound. It is an ionic compound, made up of K+ ions and IO3- ions in a 1:1 ratio. It is white crystalline powder. It is soluble in KI solution. It could be oxidized by oxygen in air, so it would be use careful in practice work and do not keep it on air.
Potassium iodate is an oxidizing agent and as such it can cause fires if in contact with combustible materials or reducing agents, so it would be use careful in practice work and do not put it near an open fireix.
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. It is white crystalline salt. Potassium iodide is ionic, K+I−. The iodide ion is a mild reducing agent, I − is easily oxidized to I2 by powerful oxidizing agents. It could be oxidized by oxygen in air and change colour to yellow, so it would be use careful in practice work and do not keep it on airx.
Method:
Method for Vitamin C:
We need to prepare 2L of Vitamin C solution using 8 tablets (1 tablet) for 250mL of distilled water).
Put 4 tablets to the beaker with distilled water. Shake the solution by glass stick.
After disappearing of tablets, put solution to flask of 1L and put more water, before it would be 1L there.
Put it to bottle of 2L.
Do that again with another 4 tablets.
Shake twice the bottle with solution.
After that, put Vitamin C solution from the bottle to six flasks, about 250mL for each (we need 5 titration of one temperature’s solution, so get 125 mL for one solution, but also we need some extra volume of solution, if we would be not accurate and split some out or if we would need some extra titrations).
Mark them from 1 to 6.
Method for temperature:
My group chooses to put Vitamin C solution separately in different temperatures, which are -20°C (in ice), 0°C (in ice), 20°C (room temperature), 40°C (water bath), 60 (water bath), 80°C (water bath) and 100°C (water bath). Because we don’t have much time, we gave up doing the experiments with Vitamin C solution at 30°C and 50°C. Before doing the experiment, we need to keep each conical flask of Vitamin C solution at the certain temperature for 15 minutes, so that temperature has enough time to affect the solution. After that we take them away and cool or warm it up to room temperature, because the cold/heat temperature decreases/increases the speed of reaction between Vitamin C and iodine particles, we can’t check the obvious correctly, the temperature makes the iodine escape, so this makes a big mistake for our data. Also too hot temperature could effect on pipet, which is made of glass. Probably, it could become warm and just crack.
Possible range: -20°C, 0°C, 20°C, 40°C, 60°C, 80°C, 100°C.
For find amount of Vitamin C, I should know how many particles (amount) of iodine (I2) reacted with Vitamin C. For knowing that I should know how many particles (amount) of iodine (I2) reacted with sodium thiosulfate (Na2S2O3) before and after reacting I2 with Vitamin C. Also there is an important thing we have to do: we need to check the exact concentration of the standard solutions such as the sodium thiosulfate (Na2S2O3*5H2O) and potassium iodide (KIO3).
During my experiment I was compelled to change time for keeping each conical flask of Vitamin C solution at the certain temperature from 15 to 10 minutes. I changed it because I had not enough time. Also I could not to get 0°C and -20°C temperatures, because I forgot to use freeze, so I had useful thing for making my solution cool is only ice. The ice was not enough to get 0°C temperature or lower, so I used 4°C is my minimum temperature. I did not use whole range of temperature: -20°C, 0°C, 20°C, 40°C, 60°C, 80°C, 100°C, because I had not enough time.
Calibrated pipette volume was 24.84mL and later 24.79mL, because previous pipette was broken. I changed my calculations for these values of Vitamin C, so it does not have effect on my data.
Controlling variables:
The quantities and concentrations were kept constant throughout the practical. Sodium thiosulfate solution was standardized to ensure correct concentration. Also before each titration, equipment was rinsed out to prevent contamination from previous uses, which could have affected the concentration/quantity of chemical.
I tried to minimize the parallax error. Every solution that I measured was at the eye level of the meniscus and the conical flask with solution stated on the table. Hence, my data should be pretty accurate.
Some other variables could affect the concentration of Vitamin C, but I tried to minimize them. For example, the exposure time of solution with Vitamin C in open air was minimized by taking out a 25mL sample of Vitamin C solution each time just before titration. At the other time, the flask with solution was closed by foil. By that I tried to minimize oxidation of Vitamin C by oxygen in air. Also it helped me to minimize effect of evaporation because, when some of the solutions were heated, they could lose water causing to increase of Vitamin C concentration.
Other example is the time exposed to light. Vitamin C is quite sensitive to UV radiation, so the strong light beams or light of room bulb can cause decomposition of Vitamin C. Therefore, I kept bottles with Vitamin C solution under tables, trying to minimize the effect of UV radiation.
Another aspect, which could have effect on the concentration of Vitamin C, is the time of stating of each conical flask in the special temperature. For that I used 10 minutes for each. It gives enough time for Vitamin C solution to have effect of temperature.
The reliability of the procedure was verified by repeating the titrations of Vitamin C and blank at least 3 more times for each temperature, and averaging of the results.
After heating or cooling of flasks we took them away and cool or warm it up to room temperature, because the cold/heat temperature decreases/increases the speed of reaction between Vitamin C and iodine particles, we can’t check the obvious correctly. Also the temperature makes the iodine to sublimate into iodine gas, so this could cause a big mistake for our data.
We made solutions with iodine exactly before each Vitamin C titration, because the iodine is sensitive to room temperature and could be sublimation into noxious gas. First of all, it is dangerous to us to make iodine solutions much early before titration. Also it could have effect on the concentration of iodine in solution (it will be decreasing) and, therefore, on the concentration of Vitamin C (it will be decreasing).
The tablets, which are used in the practice for making Vitamin C solution, are stored in a plastic opaque container/tube. This container was closed before its using, so the tablets did not have effect of UV light or humidityxi, and the concentration of Vitamin C in the tablets were not changed.
