
- •Abstract
- •Chapter 1 – Introduction
- •Chapter 2 - Methods
- •Chapter 3 - What is stroke
- •Figure 1 Post stroke disabilities (Genentech., 2014)
- •Ischaemic stroke happens when a blockage in the artery supplying the brain occurs, leading to hypoxia. Causes of ischaemic stroke include:
- •Figure 4 Molecular process of neuronal ischaemia. (Colledge, Walker and Ralston., 2010, p. 1182)
- •Available treatments for stroke
- •Figure 5 The mechanism of aspirin inhibiting ptgs enzyme (Novara., 2013)
- •Figure 6 Mechanism of t-pa action, (Genentech, 2014)
- •Embryonic stem cells
- •Figure 8 Formation of neurons from a blastocyst (Lindvall et al., 2004)
- •Adult stem cells
- •Figure 10 Reprogramming adult stem cells into induced pluripotent stem cells usig Oct4, Klf4, Sox2 and c-Myc genes (Meregalli et al., 2011).
- •Mesenchymal stem cells
- •MsCs for treatment of stroke
- •Chapter 5 - Migratory mechanisms of msCs
- •Chapter 6 - Discussion
Chapter 5 - Migratory mechanisms of msCs
Homing refers to the ability of MSCs to migrate towards the damaged area, yet the mechanisms of homing are not well understood. Whether MSCs undergo active homing, similar to endothelial-leukocyte cell adhesion mechanism (mediated by intravascular cellular adhesion, rolling and finally vascular extravasation, movement through stromal tissue and engraftment) or get passively trapped in capillaries or postcapillary venules (localization), instead of being arrested on endothelium by selectins and integrins is still not clear (Figure 8) (Karp and Leng Teo., 2009).
Biochemical messages from the damaged area, MSCs reception of them and chemotactic ability, all influence MSC migration. It is challenging to determine the exact location of MSCs following infusion, as either transendothelial migration or localization can occur (Karp and Teo., 2009).
A
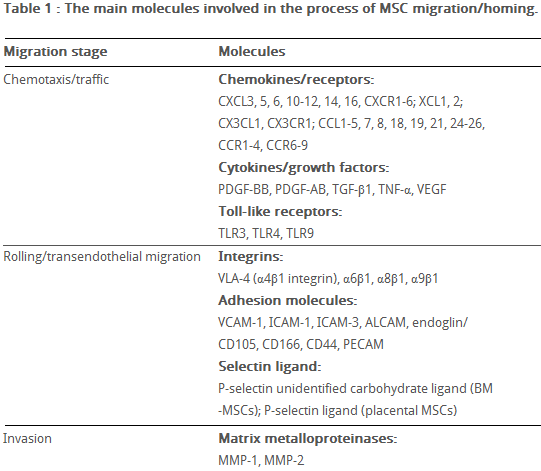
Table 6 The main molecules involved in MSC migration/homing. (Kholodenko et al., 2013)
fter tissue damage, surviving local astrocytes, microglia and endothelium up-regulate inflammatory mediators, directing the migration of MSCs towards the inflicted penubmra (Table 6) (Kholodenko et al., 2013).
Ruster et al., (2006) provide evidence for active MSC homing and state that MMP appears to be essential for MSC migration through type I collagen-rich stromal tissues. MMP-2, MMP-9 and membrane-type-1 matrix metalloproteinase (MTI-MMP) degrade components of the extracellular matrix, allowing for stem cell motility and later differentiation. The study also demonstrates MSC attachment to P-selectins on the vascular endothelium, using VLA-4 / VCAM-1 interaction, which induces strong adhesion to endothelial cells and rolling.
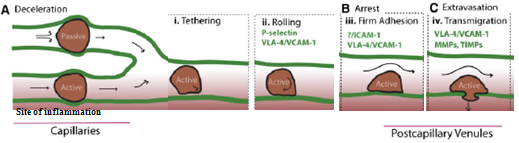
Figure 8 Model of active homing of MSCs (Karp and Leng Teo., 2009).
(A) Due to MSC large size and narrow capillaries the speed of homing is slow, which could lead to passive entrapment.
MSCs that change shape are likely to pass through the blood vessels, following a chemokine gradient towards the site of inflammation (1-tethering, 2-rolling, 3-firm endothelial adhesion, 4-extravasation followed by transendothelial migration).
(B) Adhesion molecules help MSCs flatten and stretch, attaching to the endothelial cells without affecting the blood flow when active homing occurs. In contrast, passive arrest alters blood flow and the destiny of passively arrested MSCs is unknown.
(C) MSCs transmigration occurs in acive homing.
De Becker et al., (2007) stress the importance of MSC culturing conditions, because they have a significant impact on MSC function. The migration potential is decreased if the confluence of MSCs in culture is too high, because of increased production of TIMP-3, a natural MMP inhibitor.
Liu et al., (2011) state that SDF-1 produced by damaged tissues is essential for MSC attraction. The cognate receptor for SDF-1 is CXCR4. Wynn et al., (2004) looked into CXCR4 expression on human bone-marrow derived MSCs. The results showed low (1% of cells) CXCR4 cell surface expression, yet 83% -98% of MSCs showed high level of intracellular CXCR4 expression. Shi et al., (2007) suggest that the intracellular CXCR4 can be induced to the cell surface within a few hours of in vitro stimulation with a cocktail of cytokines. Bhakta et al., (2006) also suggest that CXCR4 gene can be expressed on MSCs using retroviral transduction. In this study, MSCs were isolated from healthy volunteers, cultured and transduced with either a CXCR4 containing retroviral vector and green fluorescent protein (GFP) or only GFP - control vector. Figure 9 shows that MSCs transduced with CXCR4 migrated significantly more toward SDF-1.
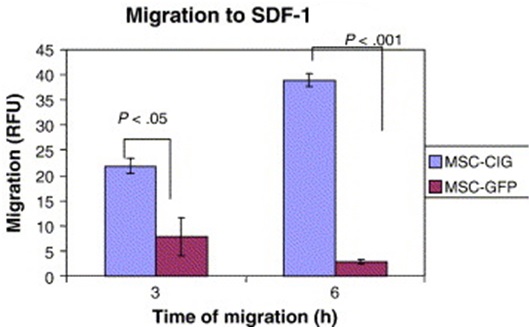
Figure 9 MSCs transduced with the CXCR4 vector, compared to MSCs transduced with the control vector (GFP) demonstrated significantly more migration to SDF-1 after both 3 and 6 hours. (Bhakta et al., 2006)
Honczarenko et al., (2006) conducted a study on in vitro-cultured, second passage MSCs, which were positive for CCR1, CCR7, CCR9, CXCR4, CXCR5, and CXCR6 cell surface receptors and secreted following chemokine ligands: CCL2, CCL4, CCL5, CCL20, CXCL12, CXCL8, CX3CL1. Some of these ligands can activate the MAP kinase and focal adhesion kinase (FAK) signaling pathways, by phosphorylation. For example, CCL5 activates STAT-1 and CXCL12 selectively triggers STAT-5 activation, inducing F-actin polymerization in the cytoskeleton. These pathways are important for transcription of integrins (VLA-4) and adhesion signalling molecules (P and E-selectins) and therefore cell migration.
In the Sackstein et al., (2008) study, MSCs did not express E-selectin ligands, but did express a CD44 cell surface receptor. In this study glycosylation of native CD44 receptor with 2,3-sialyl modifications occurred, using 1,3-fucosyltransferase to enzymatically engineer CD44 to induce E-selectin binding. CD44 was converted into hematopoietic cell E-selectin/L-selectin ligand (HCELL)6, able to induce strong E-selectin binding, which stimulates VLA-4 integrin activation, mediated by Rac1/Rap1 GTPase signaling pathway. Triggered VLA-4 adheres to VCAM-1, which mediates adhesion and signal transduction, leading to MSC transendothelial migration. (Thankamony and Sackstein., 2011).
In addition, a study by Tsai et al., (2011) has found that priming MSCs with VPA or lithium increased homing to the cerebral infarcted regions via CXCR4 and MMP-9 upregulation, and copriming with VPA and lithium further enhanced this effect.
Forte et al., (2006) also stress the importance of hepatocyte growth factor (HGF) produced by the damaged tissues. The study has show that both human and mouse MSCs can express HGF cognate receptor c-Met after brief in culture exposure to HGF. When HGF/c-Met interaction occurs in vivo, downstream transcription pathways (ERK1/2, p38MAPK and PI3K/Akt) are activated, which promote integrin synthesis favouring migration. However, prolonged exposure to HGF lead to the rearrangement of the cytoskeleton (probably due to F-actin polymerization) and the decrease in MSCs proliferation. The researchers suggest that it is because of MSC arrest in the G1-S phase. However, p38 inhibitor SB203580 was able to re-establish MSC proliferation.
This data suggests that several chemokine axes exist in MSC biology and it is important to validate MSCs intended for regenerative therapy prior to their use.
