
- •Introduction
- •Figure 1 Post stroke disabilities (Genetech 2014)
- •87% Of strokes are ischemic, the rest are hemorrhagic.
- •Available treatments for stroke
- •Induced pluripotent stem cells
- •Mesenchymal stem cells
- •Figure 8 Variety of tissue types msCs have the potential to differentiate into, depending on signals produced by the local physiological environment. (Kishk et al., 2010)
- •MsCs for treatment of stroke
- •Migratory mechanisms of msCs
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multi-potent cells with a strong capacity for self-renewal. They are capable of multilineage differentiation into mesoderm-type cells such as osteoblast, adipocyte, chondrocyte and possibly, but still controversial, other non-mesoderm type cells, for example, neuronal cells or hepatocytes. (Abdallah et al., 2008) (Figure 8)
They can be isolated from a variety of tissues, such as bone marrow, adipose tissue, umbilical cord, and umbilical cord blood. MSCs have the ability to differentiate into a variety of cell types, depending on cues from their microenvironment. MSCs are identified by the expression of many molecular markers, such as: CD105, CD73 and are negative for the hematopoietic markers CD34, CD45, CD14. (Daminici et al., 2006)
An important property of MSCs is their capacity for migration and homing in or around the zones damaged by ischaemia, inflammation, and trauma or tumour sites. Understandably, the efficacy and time course of cell invasion into the damaged tissues depends upon the route of transplantation. (Kholodenko et al., 2013)
MSCs have great potential as therapeutic agents, since they can be obtained fairly effortlessly and can be rapidly expanded ex vivo for autologous transplantation, according to Dharmasaroja et al, (2009).
Figure 8 Variety of tissue types msCs have the potential to differentiate into, depending on signals produced by the local physiological environment. (Kishk et al., 2010)
MsCs for treatment of stroke
MSC therapies include cell delivery via intravenous, intracerebral or intrathecal injection. Intravenous and intra-arterial delivery requires an open blood brain barrier (BBB) and access to the penumbra, which limits the timing of therapy to a period when the BBB is compromised. (Borlongan et al., 2011) MSCs transplanted into the brain have been demonstrated to promote functional recovery by producing trophic factors that induce survival and regeneration of host neurons, as well as alter the gap junction coupling between astrocytes, allowing them to respond more effectively damage. (Li and Chopp, 2009) The primary trophic property of MSCs is the secretion of growth factors and other chemokines to induce cell proliferation. MSCs express mitogenic proteins such as: transforming growth factor-alpha (TGF-α), TGF-β, hepatocyte growth factor (HGF), epithelial growth factor (EGF), basic fibroblast growth factor (FGF-2) and insulin-like growth factor-1 (IGF-1). (Murphy et al., 2013) (Figure 9)
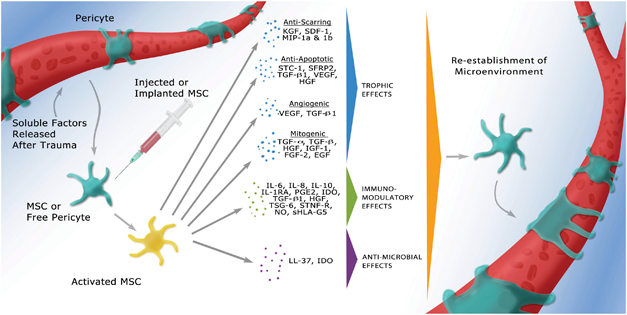
Figure 9 Activated MSCs secreting trophic, immunomodulatory or antimicrobial factors (Murphy et al., 2013)
Benefits of MSCs in the ischemic brain include: transdifferentiation, induction of neurogenesis and angiogenesis, neuroprotection, and activation of endogenous neurorestorative processes, as well as decrease apoptosis, reduce levels of free radicals, encourage synaptic connection from damaged neurons and regulate inflammation, primarily through paracrine actions. (Nanette Joyce et al, 2010) (Figure 10)
Parekkadan et al.,(2010) and Chen et al., (2001) Conclude that although the benefits of MSCs are undeniable, the homing of MSC to brain infarcted sites is far from ideal.
Cell administration into the bloodstream is more suitable for clinical use. Intra-arterial infusion may be quite effective with respect to attaining maximum cell concentration in the area supplied by a particular artery [18,19]. However, it also requires at least minor surgery and may end up with plugging of local capillaries with transplanted cells.
Iv administration is safer than the above mentioned transplantation routes and at the same time allows cell delivery to the majority of tissues. The main advantages of this method of MSC transplantation are the following: 1) availability and the absence of any contraindications, as opposed to site-directed transplantation&; 2) the possibility to inject high doses of cells for a long time, for example, using a dropper, and the ability to infuse multiple doses at certain intervals; and 3) absence of the necessity of surgical intervention. However, this method of stem cell administration has one major drawback, which lies in the fact that not all the injected cells, but rather their smaller number, reach the site of injury.
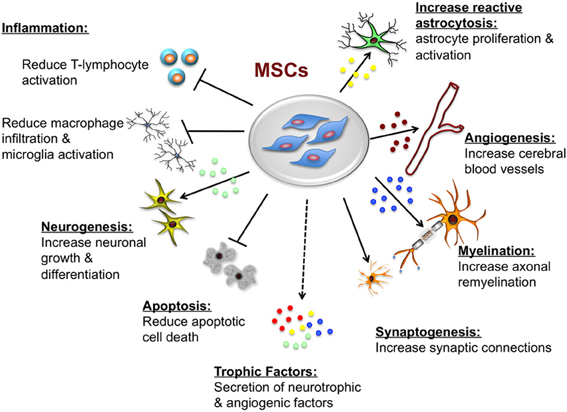
Figure 13 Potential neuroprotective and neurorestorative effects of mesenchymal stem cells. (Castillo-Melendez et al., 2013)
