- •Abstract
- •Introduction
- •Electroconductivity
- •1.2 Brief history of electric discharge research
- •Ionization
- •Electric discharges in media
- •2.1 Self-maintained and non-self-maintained discharges
- •2.2 Electric discharge in gases
- •Ionising radiation detectors
- •2.4 Glow discharge
- •2.6 Corona discharge
- •Conclusion
Electric discharges in media
2.1 Self-maintained and non-self-maintained discharges
Gas discharge can occur without external ionizer. Discharge is able to support himself. Why is this possible?
Non-self-discharge. To investigate the gas discharge at different pressures is convenient to use a glass tube with two electrodes (pic.5).
|
Picture 5. Primary gas tube |
Suppose that using a gas ionizer is forms in a certain number of pairs of second charged particles of electrons and positive ions.
When a small potential difference between the electrodes of the tube is positively charged ions migrate to the negative electrode and the negatively charged electrons and ions - the positive electrode. As a result, the tube generates an electric current, i.e., a gas discharge occurs.
Not all of the resulting ions reach the electrodes, some of them reunited with electrons to form neutral gas molecules. As the potential difference between the electrodes of the tube fraction of charged particles reaching the electrodes increases. The current in the circuit increases too. Finally, there comes a point at which all of the charged particles produced in the gas for a second, reach for this time of the electrodes. In this case the further growth of the current does not occur ( pic.6 ). Current, as they say, reaches saturation. If the action of ionizing stop, then stop and discharge, as no other ion sources . For this reason, such a discharge is called non-self- discharge.
|
Picture 6. Selfmaintained discharge’s current-voltage characteristic. |
Self-sustained discharge. What will happen with the discharge in the gas, if you continue to increase the potential difference across the electrodes?
It would seem that the current strength and a further increase in the potential difference should remain unchanged. However, experience shows that in gases at increasing the potential difference between the electrodes, from a certain value, the current increases again ( pic.7 ). This means that there are additional gas ions over and above those which are formed by the action of the ionizer. Current may increase by hundreds and thousands of times, and the number of ions produced in the discharge process can become so large that the external ionizer will no longer need to maintain the discharge. If you remove the external ionizer, the discharge stops. As the discharge in this case does not need to maintain its external ionizer, it is called self- discharge.
|
Picture 7. Non-selfmaintained discharge’s current-voltage characteristic. |
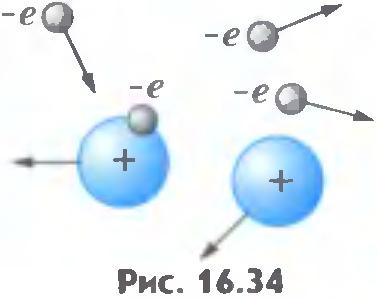
Electron impact ionization. What are the reasons for the sharp increase in the current in the gas at high voltages?
Consider a pair of charged particles (positive ion and electron), formed by the action of an external ionizer. Appeared so free electron starts to move toward the positive electrode - the anode, and the positive ion - to the cathode. On his way meets electron ions and neutral atoms. Between two successive collisions kinetic energy increases at the expense of the electric field strength. The greater the potential difference between the electrodes, the greater the intensity of the electric field.
Kinetic energy before the next collision is proportional to the length of the field and electron mean free path l (path between two successive collisions)
 (1)
(1)
If the electron kinetic energy exceeds the work Ai, you want to commit to ionize a neutral atom , ie
 (2)
(2)
self-sustained discharge
then the collision of an electron and an atom is ionized ( pic.8 ). As a result, instead of a single free electron formed two (incident on the atom and pulled out of the atom). These electrons, in turn, prepared in the energy field, and counter ionize atoms, etc. The number of the charged particles increases dramatically, electron avalanche occurs. The described process is called electron impact ionization. But only one electron impact ionization cannot provide long-self-sustained discharge. Indeed, because all appearing in this way the electrons move toward the anode and the anode reach "out of the game". For the existence of the discharge required electron emission from the cathode (emission means "emission"). Electron emission can be due to several reasons. Positive ions formed in the collision of free electrons with neutral atoms, in its motion to the cathode by the field acquire a high kinetic energy. By shocks such fast ions on the cathode surface, electrons are knocked out of the last.
|
Picture 8. Atom’s ionization. |
Furthermore, a cathode can emit electrons when heated to a high temperature. With self- heating of the cathode discharge may be due to its bombardment by positive ions that occurs, for example, an arc discharge.
In gases at high electric fields the electrons reach such high energies that begin electron impact ionization. Discharge becomes independent and continues without external ionizer.
In rarefied gas discharge occurs independently at relatively low voltages. Due to the low pressure electron mean free path between two great strikes, and he can acquire enough energy to ionize atoms. When such a discharge gas lights glow color depends on the type of gas. Glow that occurs when a glow discharge is widely uses for advertising, room illumination by fluorescent lamps [2, 7].