
3.2.2. Molecular orbital theory.
Molecular orbital theory views a molecule as a set of positive nuclei with electrons moving in the field of these nuclei. Electrons in a molecule do not necessary belong to any individual atom. The electrons occupy orbitals that extend the entire molecule called molecular orbitals (MOs). Quantity of MOs is equal to quantity of atomic orbitals they are formed from. The ground state electronic structure of a molecule is derived by placing the appropriate number of electrons into the set of MOs. The MOs are filled according to the same principles, as atomic orbitals: (1) each electron is placed into the lowest energy orbital available, (2) no more than two electrons populate a single orbital, and (3) electrons with unpaired spins are spread out as much as possible, over orbitals of the same energy.
The simplest way to visualize shapes of molecular orbitals is to consider various combinations of atomic orbitals that reside on the nuclei composing the molecule, taking into account the signs of the wave functions. Energies of molecular orbitals can be calculated from energies of atomic orbitals they are formed from.
Bonding molecular orbital. When portions of orbitals with the same sign overlap, the amplitudes of orbitals are added. The resulting molecular orbital has a shape that concentrates electron density between the two nuclei. Electrons placed in such a molecular orbital tend to hold the nuclei together and stabilize a molecule. For that reason, this orbital is called a bonding molecular orbital.
Antibonding molecular orbital. When portions of orbitals of opposite signs overlap, a molecular orbital produced has the maximum electron density outside the region between the two nuclei. If the electrons of a molecule are placed into this molecular orbital, they destabilize the molecule, therefore the orbital is said to be antibonding. Antibonding character of an orbital is denoted by an asterisk superscript (*).
Nonbonding molecular orbital. In some cases interaction of two orbitals result in appearance of two overlaps zones: one with the same signs of portions of orbitals and the second with the opposite ones. The increase of the electron density in one zone is completely compensated by the decrease of the electron density in the second one. The electrons of a molecule placed into this molecular orbital, neither stabilize, nor destabilize the molecule, therefore the orbital is said to be nonbonding.
The net bond order can be defined as:
Net bond order = |
(number of e in bonding MOs – number of e in antibonding MOs |
2 |
Electrons, placed into the bonding molecular orbital lead to stable bond formation and, therefore, energy of this orbital is lower than that of the two initial atomic orbitals. On the other hand, electrons, placed into the antibonding orbital lead to destabilization of the molecule and thus to state higher in energy than that of the two initial atomic orbitals. This can be represented schematically, as shown in Fig. 3.5, where the energies of the atomic orbitals of the separate appear on both sides of the energy-level diagram and the energies of the molecular orbitals are placed in the center. Using this simplest diagram we can examine the bonding in the H2 molecule (Fig. 3.5). There are two electrons in H2 that we place in the lowest-energy molecular orbital, the 1s. Using the same diagram, we can see why the molecule He2 does not exist. The species would have four electrons, two of which would be placed into the 1s orbital and. The other two would be forced to occupy the *1s orbital. As a result, the net bond order has a value of zero for He2. Since the bond order is zero in He2, the molecule doesn’t exist. Thus, the net bond order in molecular ion He2+ is equal to 0.5, this particle may exists in appropriate external conditions.
For diatomic molecules of second period elements, only molecular orbitals derived from the interaction of the valent shell 2s and 2p orbitals should be considered. The 1s orbitals are not involved to any appreciable extent in the bonding in these molecules.
The real mark of success for the molecular orbital theory is an adequate description of the O2 molecule. This molecule is found experimentally to be paramagnetic with two unpaired electrons. In addition, its bond length and bond energy suggest, that there is a double bond between the two oxygen atoms. Paramagnetic properties of the O2 molecule can’t be explained by a valence bond theory; an attempt gives us (1) a molecule with a double bond and all the electrons paired, (2) a molecule containing two unpaired electrons, but oxygen are bonded by single O-O bond only. The molecular orbital description of O2 is shown in Fig 3.6. The first 10 of 12 valence electrons populate all the same molecular orbitals, as in N2. The final 2 electrons must then be placed in the *2px and *2py antibonding orbitals. As these orbitals are of the same energy, electrons spread themselves out with their spins in the same directions. These two antibonding electrons cancel the effect of the two of the -bonding electrons, so the net bond order in O2 has a value of two. So, for O2 molecule predictions of the MO theory are in precise agreement with experimental evidence (both bond order and quantity of unpaired electrons is predicted correctly).
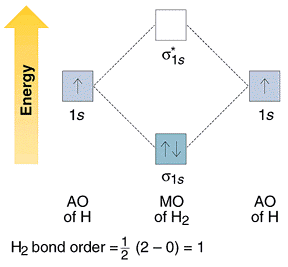
Fig. 3.5 Bonding in the H2 molecule.

Fig. 3.6 Bonding in the O2 molecule
Polar covalent bond. If the two atoms joined by the covalent bond differ in electronegativity, the electron pair will be pulled more towards the atom with the higher electronegativity. For example, consider the HCl molecule. Chlorine is more electronegative, than hydrogen; therefore, more than half of the electron density of the bond pair is concentrated around the chlorine atom. As a result, the chlorine atom is negatively charged and the hydrogen atom is positively charged in the HCl molecule. However, electron transfer from H to Cl is not complete, and HCl is far from being ionic compound. The measurement suggest, that these charges are only about +0.17 on the hydrogen atom and –0.17 on the chlorine one. (The partial charges on atoms are usually indicated by lowercase Greek letter delta: + and -). Equal negative and positive charges separated by a distance constitute a dipole. Thus, the HCl molecule is a dipole and is said to be polar. A dipole is defined quantitatively by its dipole moment, the product of the charge on either end of the dipole and the distance between the charges. A bond with a large dipole moment is said to be very polar, while nonpolar bond will have no dipole moment at all.
There is no sharp dividing between covalent and ionic substances. Even in compounds that we think of being ionic, such as NaCl, there is some degree of covalent character of the bonds between atoms. As a very rough guide, bonds become more than 50% ionic when the electronegativity difference between the atoms is lager than about 1.7 and we normally consider substances with these bonds as being ionic compounds.
