
- •Lesson 2 Grammar: the Present Indefinite Tense Where to find rocks?
- •Where do rocks come from?
- •Lesson 3 differences between rocks Rock textures
- •Lesson 4 Grammar: the Past Indefinite Tense
- •Investigating how porous rocks are
- •Acid rain (1)
- •Acid rain (2)
- •Lesson 6 Grammar: The Future Indefinite Tense the effect of temperature change
- •By daily expansion and contraction.
- •By freeze-thaw damage
- •Transportation of fragments
- •Lesson 8 Grammar: Future actions in when-, if- clauses Flooding and sediment formation
- •Lesson 9 Transport of sediments
- •Lesson 10 Grammar: The plural of nouns
- •Pollution
- •Lesson11
- •Lesson 12
- •Lesson 13 (revision)
- •Erosion of rocks
Lesson 4 Grammar: the Past Indefinite Tense
Investigating how porous rocks are
A rock from group (a) and a rock from group (b) were placed in water. They were weighed before and afterwards and two observations were made.
rock (a) rock (b)
A
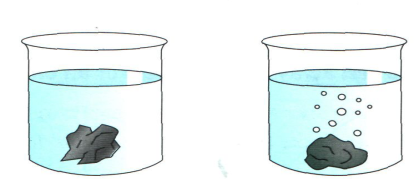 ir
bubbles were lost from rock (b) due to air escaping.
ir
bubbles were lost from rock (b) due to air escaping.Rock (b)gained more mass as the air spaces were filled with water.
(b)
Original mass 63.1g 52.9g
“Wet” mass 64.7g 64.3g
Change in mass 1.6g 11.4g
Remember the words:
investigate – досліджувати to weigh – зважувати weight – вага observation – спостереження to lose – втрачати to gain – набувати, діставати crack – тріщина sample – зразок to escape – тікати, виділятися (про газ)
Answer the questions:
What was the experiment made for?
What kinds of rocks were taken for the experiment?
Where were the samples of rocks placed?
When were the samples of rock weighed?
Why were air bubbles lost from rock (b)?
Why did rock (b) gain more mass?
As we already know, the grains are interlocking in the rocks of group (a) with no spaces in between the grains. Why did rock (a) gain some mass though?
Exercise1. You missed the lesson when porous and non-porous rocks were investigated. Ask your fellow students all possible questions about the experiment.
For example: 1. What did you investigate at the lesson?
Exercise 2. Write a report how you made the experiment on porous and non-porous rocks.
Lesson 5
Grammar: The past Indefinite tense
ROCKS AND WEATHERING. WEATHERING PROCESSES
The effect of rain
The rocks at the Earth's surface are constantly exposed to rain. This rain is often slightly acidic. This is due partly to the presence of carbon dioxide in the atmosphere, which dissolves naturally in the falling rain, to produce a dilute acid. However, sulphur dioxide from industrial processes and nitrogen oxides from car exhausts also dissolve in rain, to produce acids and this can make things worse...
If acid rain falls onto CARBONATE rocks such as limestone, then a chemical reaction occurs and this produces carbon dioxide.
On other rocks the chemical reaction may not be quite so vigorous but over many years, the rocks are gradually eroded.
This can be seen in statues and on buildings, which are open to the elements. Eventually quite ornate statues and mouldings on buildings will lose their 'sharpness' as they become shapeless blobs.
Remember the words:
expose – піддавати (чомусь - to) acid – кислота
due to – завдяки carbon dioxide – двоокис вуглецю
to dissolve – розчиняти(ся) sulphur dioxide – двоокис сірки
dilute – розбавлений nitrogen oxide – окис азоту
car exhaust – вихлоп автомобіля carbonate rocks – карбонатні породи
to occur – траплятися to produce – виробляти
vigorous – сильний to erode – розмивати, вивітрювати, еродувати
ornate – пишно прикрашений moulding – ліпна прикраса, карниз
Answer the questions:
What are rocks on the earth’s surface constantly exposed to?
Why is the rain slightly acidic?
How is a dilute acid in the atmosphere produced?
Where does sulphur dioxide come from?
Where does nitrogen oxide come from?
How do these chemicals produce acids?
What happens when acid rain falls onto carbonate rocks?
Where can we see the results of erosion by acid rain?
Exercise 1. Rewrite the second paragraph of the text in the Past Indefinite tense. Make up questions and let your fellow students answer.
