
- •(Примеры химических соединений)
- •Примеры химических соединений
- •Классификация химических соединений
- •Органических соединений
- •Неорганических соединений
- •Органические соединения
- •Углеводороды
- •Ароматические углеводороды
- •Органические соединения, содержащие кислород
- •Альдегиды
- •Карбоновые кислоты
- •Неорганические соединения
- •Ионные соединения
- •Молекулярные соединения
- •Водные растворы кислот
- •Анотация
The Ministry of Education and science
Donetsk National University
Report
Examples of Chemical Compounds
(Примеры химических соединений)
Submitted by
Irina Galutskaya
1-st year student
The department of chemistry
Language adviser
L.V.Sulina
Donetsk
2013
Examples of Chemical Compounds
Chemical compounds are substances that are made up of atoms of two or more different elements. There are millions of known compounds formed from various elements that exist in nature. The elements combine in a fixed ratio to form a specific chemical compound, and the constituent atoms are held together by chemical bonds. The composition or the ratio in which the elements are present in the compound, plays a key role in determining the properties of the compound. The compounds are named according to the rules decided by the International Union of Pure and Applied Chemistry (IUPAC).
Classification of Chemical Compounds
Chemical compounds can be broadly classified into two categories, namely, organic compounds and inorganic compounds. While organic compounds are further classified on the basis of the functional group present, inorganic compounds are classified on the basis of the type of bonds between the constituent atoms. Here we shall look at some examples of compounds under each category.
Types of Chemical Compounds
Organic Compounds
• Hydrocarbons
• Compounds Containing Oxygen
• Compounds Containing Nitrogen
Inorganic Compounds
• Ionic compounds
• Molecular compounds
• Aqueous acids
Organic Compounds
Organic compounds are complex compounds of carbon in which one or more atoms of carbon are covalently linked to atoms of other elements, such as hydrogen, nitrogen, and oxygen. Hydrocarbons are organic compounds that are made up of carbon and hydrogen atoms only. When functional groups are attached to one or more carbon atoms of the hydrocarbon chain, then the chemical properties change. Organic compounds are classified on the basis of the functional group attached to the carbon atoms.
Hydrocarbons
H
R-H
Alkane
ydrocarbons are further classified into alkanes, alkenes, alkynes, and aromatic hydrocarbons. Let us see some examples of each.
Alkanes
Alkanes are saturated hydrocarbons having single bonds between each pair of carbon atoms. The simplest alkane is methane, in which one carbon atom is linked with four atoms of hydrogen. Ethane comes next, and has two carbon atoms linked to each other by single covalent bonds. Alkanes are the least reactive hydrocarbons. The representation of alkanes is shown alongside. The general formula for alkanes is CnH2n+2, while the same for cyclic alkanes is CnH2n.
Examples
• Propane (C3H8) • Cyclohexane (C6H12)
• Butane (C4H10) • Cyclopentane (C5H10)
• Pentane (C5H12) • Decane (C10H22)
A
C=C
Alkene
lkenes
Alkenes are hydrocarbons having at least one C=C double bond. The simplest alkene is ethene, in which two carbon atoms are linked with a double covalent bond. Alkenes exhibitcis-trans isomerism, and are more reactive than alkanes. The general formula for alkenes with a single double-bond is CnH2n, while the same for cyclic alkenes is CnH2n-2. Alkenes with more than one double bond, are known as polyenes.
Examples
• Propene (C3H6) • Octene (C8H16)
• Butene (C4H8) • Cyclopentene (C5H8)
• Pentene (C5H10) • Cyclohexene (C6H10)
• Hexene (C6H12) • 2-Methylpropene (C4H8)
•
C≡C
Alkyne
Heptene (C7H14) • 2-Methyl-2-hexene (C7H14)
Alkynes
Alkynes are hydrocarbons having at least one C≡C triple bond. The simplest alkyne is ethyne, in which two carbon atoms are linked with a triple covalent bond. Alkynes are the most reactive hydrocarbons. The general formula for alkynes is CnH2n-2, while the same for cyclic alkynes is CnH2n-4.
Examples
• Propyne (C3H4) • Cyclooctyne (C8H12)
• Butyne (C4H6) • 1-hexyne (C6H10)
• 4-methyl-2-pentyne (C6H10) • 1-heptyne (C7H12)
• 4-methyl-1-pentyne (C6H10) • 1-decyne (C10H18)
• 1-pentyne (C5H8) • 5-cyclopropyl-1-pentyne (C8H12)
Aromatic
Hydrocarbons

Also known as arenes, aromatic hydrocarbons are characterized by a six-carbon ring that has alternate C=C double bonds, separated by C-C single bonds. They are known as aromatichydrocarbons because most of these compounds have a sweet scent. Benzene is the simplest of these compounds. There are two types of aromatic hydrocarbons: monocyclic aromatic hydrocarbons (MAH) and polycyclic aromatic hydrocarbons (PAH).
Examples
• Methylbenzene or Toluene (C7H8) • 1,3,5-trimethylbenzene (C9H12)
• Styrene (C8H8) • Ethylbenzene (C8H10)
Organic Compounds Containing Oxygen
There are many functional groups that contain one or more oxygen atoms. Given below is a list of such functional groups, along with examples of compounds under each.
Hydroxyl
Group ![]()
The hydroxyl group is a functional group that consists of a hydrogen atom and an oxygen atom, linked together by a covalent bond. The anion (OH-) is the hydroxide anion, in which the negative charge resides on the oxygen atom. The hydroxyl radical (HO) is the neutral form of the hydroxyl group. In organic chemistry, the hydroxyl group is the defining functional group in alcohols. The general formula for acyclic alcohols is CnH2n+1OH.
Now, let us have a look at some examples of organic compounds with the hydroxyl group.
Examples
• Methanol (CH4O) • Butanol (C4H10O)
• Ethanol (C2H6O) • Pentanol (C5H12O)
• Propanol (C3H8O) • Hexanol (C6H14O)
Ethers
E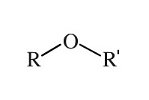 thers
are organic compounds that contain the ether group, which is
identified as two alkyl or aryl groups connected by an oxygen atom.
The general formula of ethers is R-O-R'. Depending on whether the
alkyl or aryl groups on both sides of the oxygen atom, are similar or
different, ethers are classified as simple (symmetrical) and mixed
(asymmetrical). In simple ethers, both R and R' are the same, while
in mixed ethers, R and R' are different alkyl or aryl groups. The
simplest ether is dimethyl ether, in which two methyl groups are
connected to an oxygen atom.
thers
are organic compounds that contain the ether group, which is
identified as two alkyl or aryl groups connected by an oxygen atom.
The general formula of ethers is R-O-R'. Depending on whether the
alkyl or aryl groups on both sides of the oxygen atom, are similar or
different, ethers are classified as simple (symmetrical) and mixed
(asymmetrical). In simple ethers, both R and R' are the same, while
in mixed ethers, R and R' are different alkyl or aryl groups. The
simplest ether is dimethyl ether, in which two methyl groups are
connected to an oxygen atom.
Examples
• Methoxymethane or dimethyl ether (C2H6O) • 1,4-Dioxane (C4H8O2)
• Ethoxyethane or diethyl ether (C4H10O) • Polyethylene glycol (C2nH4n+2On+1)
• Oxolane or tetrahydrofuran (C4H8O)
Aldehydes
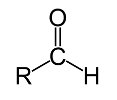
An organic compound that contains a formyl group, is known as an aldehyde. The presence of a carbonyl center that is linked to a hydrogen atom on one side, and an R- group on the other, is what defines an aldehyde. The carbonyl center is a carbon atom that is linked to an oxygen atom by double covalent bonds. The general formula for aldehydes is R-CHO, where -CHO is the formyl group. Organic compounds that have two aldehyde groups are known as dialdehydes. An example of a dialdehyde is glyoxal.
Examples
• Methanal or formaldehyde (CH2O)
• Ethanal or acetaldehyde (C2H4O)
• Benzaldehyde (C7H6O)
• Tolualdehyde (C8H8O)
• Butanal (C4H8O)
Carboxylic
Acids
Carboxylic acids are organic compounds with at least one carboxyl group. The carboxyl group consists of a carbonyl group (RR'C=O), and a hydroxyl group (R-O-H). It is represented as -COOH. The general formula for carboxylic acids is R-COOH. These are the most commonly occurring acids in organic chemistry. However, carboxylic acids are weak acids with strong odors. The simplest member of this group is methanoic acid, which is also known as formic acid.
Examples
• Formic acid or methanoic acid (CH2O2) • Pentanoic acid or valeric acid (C5H10O2)
• Acetic acid or ethanoic acid (C2H4O2) • Butanoic acid (C4H8O2)
• Benzoic acid (C7H6O2) • Toluic acid (C8H8O2)
Esters
I n
inorganic chemistry, alkalis react with acids to form salts.
Similarly, in organic chemistry, hydroxyl compounds (alcohols and
phenols) react with oxoacids (acids that contain oxygen) to form
esters. Thus, esters are nothing but salts of alcohols and acids. The
most common esters are those derived from carboxylic acids. The
process by which carboxylic acids react with alcohols in the presence
of hydrochloric or sulfuric acids, is termed as esterification. The
reaction involves the replacement of the hydroxyl (OH) group of the
acid with the alkoxy (R'O) group of the alcohol. Esters can also be
formed from acid anhydrides, acyl chlorides, carboxylate salts, and
other esters.
n
inorganic chemistry, alkalis react with acids to form salts.
Similarly, in organic chemistry, hydroxyl compounds (alcohols and
phenols) react with oxoacids (acids that contain oxygen) to form
esters. Thus, esters are nothing but salts of alcohols and acids. The
most common esters are those derived from carboxylic acids. The
process by which carboxylic acids react with alcohols in the presence
of hydrochloric or sulfuric acids, is termed as esterification. The
reaction involves the replacement of the hydroxyl (OH) group of the
acid with the alkoxy (R'O) group of the alcohol. Esters can also be
formed from acid anhydrides, acyl chlorides, carboxylate salts, and
other esters.
Examples
• Butyl butanoate (C8H16O2) • Methyl benzoate (C8H8O2)
• Ethyl acetate (C4H8O2) • Methyl pentanoate (C6H12O2)
• Ethyl formate (C3H6O2) • Ethyl pentanoate (C7H14O2)
• Isopropyl acetate (C5H10O2) • Benzyl acetate (C9H10O2)
• Isobutyl acetate (C6H12O2) • Ethyl hexanoate (C8H16O2)
Compounds Containing Nitrogen
These are compounds with a functional group that has nitrogen atoms in it, which includes amines, amides.
Amines
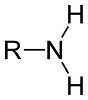
Amines are organic compounds that are derivatives of ammonia (NH3), in which the hydrogen atoms of ammonia have been replaced by alkyl or aryl groups. Amines can be identified by the presence of a nitrogen atom with a lone pair of electrons. Amines that have an aromatic ring attached to the nitrogen atom are known as aromatic amines, while others are known as aliphatic amines. All amines are more basic, when compared to ammonia. Amines can be categorized into three classes, and these are as follows:
Primary Amines
In primary amines, one of the three hydrogen atoms in ammonia, is replaced by an alkyl or aryl group. Thus, primary amines can be aliphatic or aromatic. The general chemical formula for primary amines is RNH2, and they are named as "alkylamine". Examples of aliphatic primary amines are methylamine, ethylamine, and propylamine, while an example of aromatic primary amines is phenylamine (aniline).
Secondary Amines
In secondary amines, two of the three hydrogen atoms in ammonia, are replaced by alkyl or aryl groups. The general formula for secondary amines is R2NH, and they are named as "dialkylamine". Secondary amines can be cyclic. Examples of secondary aliphatic amines are dimethylamine, ethylmethylamine, and diethylamine, while an example of secondary aromatic amines is diphenylamine.
Tertiary Amines
In tertiary amines, all the three hydrogen atoms of ammonia, are replaced by alkyl or aryl groups. In other words, a tertiary amine has three hydrocarbon groups attached to the nitrogen atom. Tertiary amines can be cyclic. The general formula for tertiary amines is R3N, and they are named as "trialkylamine". Examples are trimethylamine and triphenylamine.
If all four hydrogen atoms of an ammonium ion are replaced with alkyl or aryl groups, then a quaternary ammonium salt is formed, which is ionic. The simplest example of such a salt is tetramethylammonium chloride (CH3)4N+ Cl-. Given below are a few examples of primary, secondary, and tertiary amines.
Examples
• Propylamine (C3H9N) • Diethylamine (C4H11N)
• Butylamine (C4H11N) • Methylpropylamine (C4H11N)
• Pentylamine (C5H13N) • Diphenylamine (C12H11N)
• Phenylamine or aniline (C6H7N) • Trimethylamine (C3H9N)
• Dimethylamine (C2H7N) • Triphenylamine (C18H15N)
Amides
A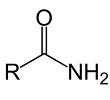 mides
or acid amides are carboxylic acid (RCOOH) derivatives, in which the
-OH part of the acid is replaced by -NH2 group. The general formula
for organic amides is RCONH2. Amides are further classified into
primary, secondary and tertiary amides, based on the number of
hydrogen atoms attached to the nitrogen atom of the functional group
-CONH2. Given below are a few examples of amides.
mides
or acid amides are carboxylic acid (RCOOH) derivatives, in which the
-OH part of the acid is replaced by -NH2 group. The general formula
for organic amides is RCONH2. Amides are further classified into
primary, secondary and tertiary amides, based on the number of
hydrogen atoms attached to the nitrogen atom of the functional group
-CONH2. Given below are a few examples of amides.
Examples
• Methanamide (CH3NO)
• Ethanamide (C2H5NO)
• Propanamide (C3H7NO)
• Pentanamide (C5H9NO)
• Benzamide (C7H7NO)
Inorganic Compounds
Inorganic compounds are compounds that do not contain carbon. However, there are exceptions, which include compounds such as carbon dioxide, carbon monoxide, etc. Inorganic compounds can be classified into ionic compounds, molecular compounds, and aqueous acids.
Ionic Compounds
Ionic compounds are compounds that contain one metal ion and one or more non-metal ions. Ionic compounds can be binary or ternary, and these two types are explained below, along with examples for each.
Binary Ionic Compounds
Ionic compounds that contain two elements, of which one is a metal and the other is a non-metal, are known as binary ionic compounds. One example of such a compound is sodium chloride (NaCl), which contains one sodium ion (metal) and one chloride ion (non-metal). More examples of binary ionic compounds are given below.
• Lithium Fluoride (LiF) • Cesium Fluoride (CsF)
• Lithium Bromide (LiBr) • Beryllium Sulfide (BeS)
• Lithium Selenide (Li2Se) • Magnesium Fluoride (MgF2)
• Lithium Nitride (Li3N) • Magnesium Chloride (MgCl2)
• Sodium Chloride (NaCl) • Calcium Bromide (CaBr2)
Ternary Ionic Compounds
Ionic compounds that contain three elements, which consist of at least one metal and one non-metal, are known as ternary ionic compounds. One example of such a compound is calcium carbonate (CaCO3), which has one calcium ion (metal), one carbon ion (non-metal), and three oxygen ions. More examples of ternary ionic compounds are given below.
Examples
• Lithium Hydroxide (LiH) • Silver Hydroxide (AgOH)
• Sodium Nitrate (NaNO3) • Silver Nitrate (AgNO3)
• Sodium Chlorate (NaClO3) • Beryllium Sulfate (BeSO4)
• Silver Peroxide (Ag2O2) • Cesium Hydroxide (CsOH)
• Magnesium Carbonate (MgCO3) • Magnesium Sulfate (MgSO4)
Molecular Compounds
Molecular compounds are compounds in which two or more non-metal atoms form a molecule. These are also known as covalent compounds because the bonds between the atoms in the molecule are covalent bonds. Molecular compounds may be polar or non-polar, depending on the shape of the molecule, and the polarity of the bonds formed between the constituent atoms. Here are some examples of molecular compounds.
Examples
• Sulfur Dichloride (SCl2) • Boron trifluoride (BF3)
• Silicon tetrafluoride (SiF4) • Carbon tetrabromide (CBr4)
• Water (H2O) • Ammonia (NH3)
• Sulfur dibromide (SBr2) • Silicon dioxide (SiO2)
Aqueous Acids
The term aqueous is used for the solution of a substance in water. Thus, aqueous acids are acids in solution with water. These acids are either binary acids or ternary acids. Let's have a look at the examples of each.
Binary Acids
Binary acids contain two elements, of which one is hydrogen, and the other one is a non-metal. One example is hydrochloric acid (HCl), which contains one hydrogen atom and one chlorine atom (non-metal). More examples of binary aqueous acids are given below.
Examples
• Hydrofluoric acid (HF) • Hydrobromic acid (HBr)
• Hydrocarbonic acid (H4C) • Hydroiodic acid (HI)
• Hydrosulfuric acid (H2S)
Ternary Acids
Ternary acids contain three elements, of which one is hydrogen, one is oxygen, and the third element is a non-metal. One example is sulfuric acid (H2SO4), which contains hydrogen, sulfur (non-metal), and oxygen. More examples of ternary aqueous acids are given below.
Examples
• Nitric acid (HNO3) • Permanganic acid (HMnO4)
• Phosphoric acid (H3PO4) • Arsenic acid (H3AsO4)
• Boric acid (H3BO3) • Arsenous acid (H3AsO3)
• Carbonic acid (H2CO3) • Hypochlorous acid (HClO)
• Nitrous acid (HNO2) • Perchloric acid (HClO4)
These were some examples of compounds in chemistry. There are millions of chemical compounds out there and hence, it is a difficult to enlist all of them here. However, most of the compounds mentioned above, have wide applications in different areas.
