
PART1: MULTIPLE CHOICE QUESTIONS
Instructions:
For each question there are four possible answers A, B, C, D and E .Choose the one you consider the best and correct.
Record your choice in soft pencil or pen on the separate Answer Sheet.
Begin Here:
A sample of an ideal gas is held in an insulated container and it undergoes an adiabatic change. The graph below shows the change in pressure p with change in volume V as the gas changes from X to Y.
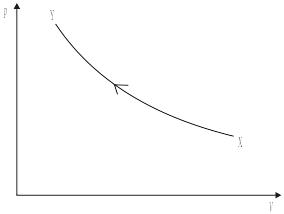
Which of the following describes correctly the work done and the change in the internal energy of the gas?
-
Work done
Internal energy
on the gas
increases
on the gas
decreases
by the gas
decreases
by the gas
increases
Work done is Zero
Internal Energy is Zero
(1)
The temperature of an ideal gas is reduced. Which one of the following statements is true?
-
The molecules collide with the walls of the container less frequently.
The molecules collide with each other more frequently.
The time of contact between the molecules and the wall is reduced.
The time of contact between molecules is increased.
none of the above
(1)
In one cycle of a heat engine, 300 J of energy is absorbed and 200 J of energy is ejected. The efficiency of the engine is
-
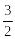



1
(1)
The graph below shows the variation with volume V of the pressure p of a gas during one cycle of an engine.
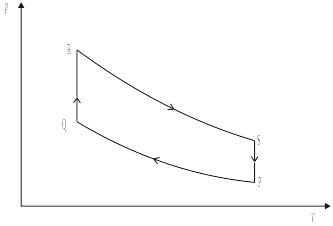
During which operations, PQ, QR, RS and SP does the gas do external work?
-
PQ only
RS only
QR and RS only
PQ and RS only
None of the above is correct
(1)
A gas is contained in a cylinder fitted with a piston as shown below.
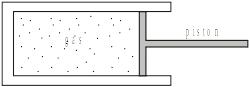
When the gas is compressed rapidly by the piston its temperature rises because the molecules of the gas
-
are squeezed closer together.
collide with each other more frequently.
collide with the walls of the container more frequently.
gain energy from the moving piston.
increase its volume.
(1)
For a system that undergoes a small change of state,
Q = U + W
where +Q = thermal energy transferred to the system
+U = increase in internal energy of the system
+W = the work done by the system.
In an adiabatic compression of an ideal gas, which one of the following is true in respect of Q, U and W?
-
Q
U
W
Zero
Positive
Negative
Zero
Negative
Negative
Positive
Positive
Positive
Negative
Zero
Positive
Negative
Positive
Zero
(1)
