
aer-03-123 (1)
.pdf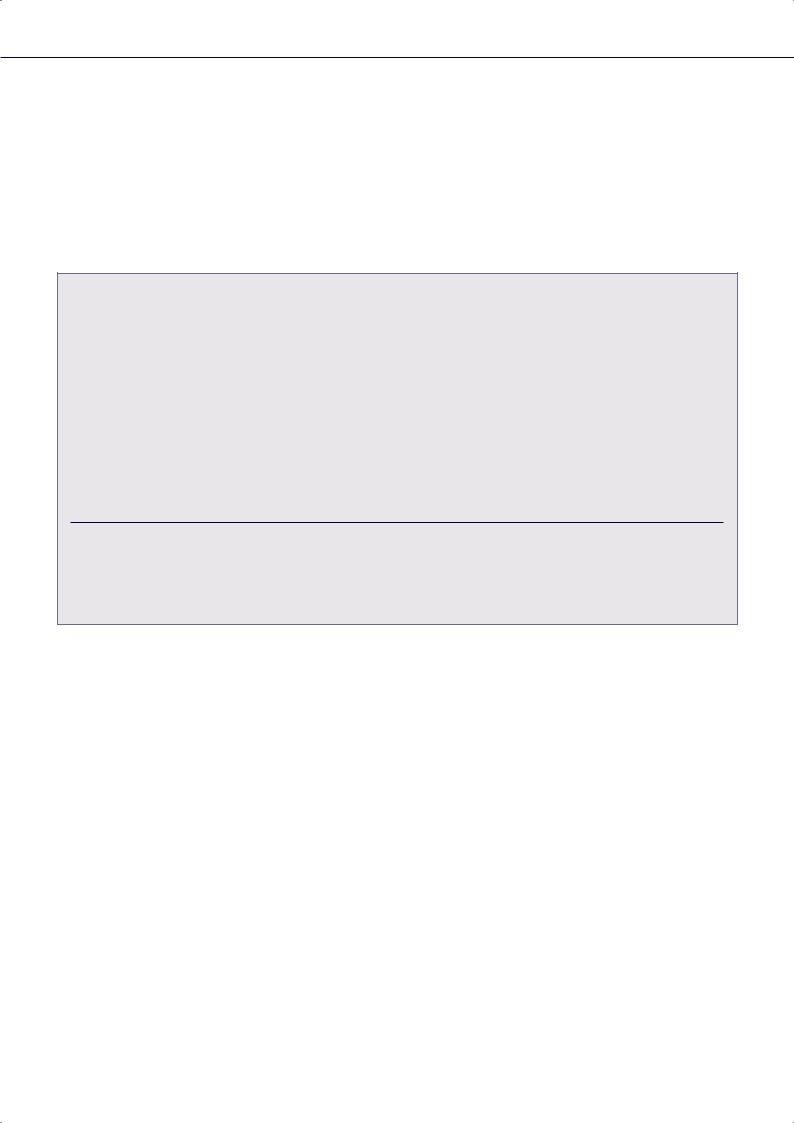
Device Therapy
Remote Monitoring for Follow-up of Patients with
Cardiac Implantable Electronic Devices
Renato Pietro Ricci,1 Loredana Morichelli1 and Niraj Varma2
1. Department of Cardiology, San Filippo Neri Hospital, Rome, Italy; 2. Cardiac Pacing and Electrophysiology, Cleveland Clinic, Cleveland, Ohio, US
Abstract
Follow-up of patients with cardiac implantable electronic devices is challenging due to the increasing number and technical complexity of devices coupled to increasing clinical complexity of patients. Remote monitoring (RM) offers the opportunity to optimise clinic workflow and to improve device monitoring and patient management. Several randomised clinical trials and registries have demonstrated that RM may reduce number of hospital visits, time required for patient follow-up, physician and nurse time, hospital and social costs. Furthermore, patient retention and adherence to follow-up schedule are significantly improved by RM. Continuous wireless monitoring of data stored in the device memory with automatic alerts allows early detection of device malfunctions and of events requiring clinical reaction, such as atrial fibrillation, ventricular arrhythmias and heart failure. Early reaction may improve patient outcome. RM is easy to use and patients showed a high level of acceptance and satisfaction. Implementing RM in daily practice may require changes in clinic workflow. To this purpose, new organisational models have been introduced. In spite of a favourable cost:benefit ratio, RM reimbursement still represents an issue in several European countries.
Keywords
Cardiac implantable electronic devices, implantable cardioverter defibrillator, remote monitoring, follow-up, recall management, atrial fibrillation, ventricular arrhythmias, heart failure
Disclosure: Renato Pietro Ricci has received minor consultancy fees from Medtronic and Biotronik; Loredana Morichelli has received minor consultancy fees from Medtronic; Niraj Varma was a consultant to Biotronik for and principal investigator of the TRUST Trial and has received minor consultancy fees from St Jude, Medtronic, Boston Scientific and Sorin.
Received: 24 July 2014 Accepted: 7 August 2014 Citation: Arrhythmia & Electrophysiology Review 2014;3(2):123–8 Access at: www.AERjournal.com Correspondence: Renato Pietro Ricci, Department of Cardiology, San Filippo Neri Hospital, Via Martinotti, 20 00135 Rome, Italy; El: renatopietroricci@tin.it
Monitoring after implant of patients with cardiac implantable electronic devices (CIED) forms a part of both device and patient care, and is the responsibility of the implanting centre. Monitoring is challenged by the increasing number and technical complexity of implanted devices coupled with the increasing clinical complexity of the patient population. Current practice is based on quarterly to yearly in-office visits with an increased rate when the device approaches its end of service, or in case of advisories.1–3 In this model, download of data stored in the device memory, potentially useful for patient management, is delayed. Telemedicine offers a unique opportunity to optimise clinic workflow and to improve device monitoring and patient management.
Technology
All major CIED manufacturers have introduced remote monitoring (RM) systems.4 All of them are based on a patient unit capable of interrogating the device and downloading the programmed parameters and the diagnostic data. Information is transmitted to a central database where it is decrypted and stored on a secure website on which it can be viewed by the clinical staff. Early wand-based systems required patient-driven downloads relayed via telephone connections to following facilities. Currently available systems are based on automatic transmission mechanisms that are fully independent of patient or physician interaction. A distinction has to be made between ‘remote interrogation’ and ‘remote monitoring’. In
the first, device interrogation is performed periodically at home either by the patient manually or by the monitoring system automatically at predefined intervals. In the second, continuous device monitoring may trigger unplanned transmissions in case of programmable alerts. In spite of major commonalities, different proprietary systems may differ substantially in connectivity, patient involvement, transmission scheduling and alert availability and programming.
Impact on Out-patient Clinic Workload
The Lumos-T Safely Reduces Routine Office Device Follow-up Trial (TRUST)5 was the first large randomised trial which demonstrated that RM may safely reduce the number of in-hospital visits by nearly 50 %. The reduction primarily occurred in scheduled encounters involving collection of routine measurements and requiring no clinical intervention. Unscheduled visits slightly increased in the remote arm. The overall reduction in face-to-face visits was obtained safely, with no difference between the two study arms in mortality, incidence of strokes and events requiring surgical interventions. Furthermore, detection of arrhythmia onset was anticipated by more than 30 days. Regarding patient adherence to follow-up, the TRUST results6 showed that patients in the RM arm more effectively and durably attained follow-up goals of punctual scheduled follow-up and patient retention compared with conventional methods. Several studies have confirmed such results and there is now strong evidence that RM significantly reduces in-hospital visit numbers, time required for patient follow-up,
© R A D C L I F F E C A R D I O L O G Y 2 0 1 4 |
123 |
|
|

Device Therapy
Figure 1: Early Detection of High Voltage Lead Failure by Continuous Remote Monitoring
Top: intermittent sudden drop in right ventricular (RV) pacing impedance. Bottom: inappropriate detection of self-limited ventricular fibrillation (VF) due to oversensing of spurious signals (noise). Combined data allowed lead replacement before any symptom experienced by the patient. Marker channel: VF = ventricular event sensed in the VF detection window; VS = ventricular sensed event; AS = atrial sensed event. FU = in-person follow-up.
Figure 2: Alert for Newly Detected Atrial Fibrillation – Internal Atrial Electrogram Confirms Appropriate Detection
AS = atrial sensed event; AP = atrial paced event; VS = ventricular sensed event; VP = ventricular paced event; AMS = automatic mode switch.
physician and nurse time and hospital costs.7–11 Furthermore, social costs for patients and caregivers which include travelling, missed work and social activities may be reduced by RM.12,13
Device Management
Lead and device performance monitoring is a physician responsibility and represents a challenge due to the number and complexity of
implanted systems and the increased number of advisories during the last 10 years. A strategy based on frequent in-hospital follow-up visits is unsuitable as it places a huge work burden on out-patient clinics. It is also inefficient since malfunctions are rare and likely to be missed when developing suddenly in the interval between visits, with the possibility of causing potentially life-threatening complications.14 RM allows continuous monitoring, with automatic alerts of battery voltage and impedance, circuit status, charging time, lowand highvoltage lead impedances, sensing and pacing threshold values, external interferences, inappropriate detection of arrhythmias due to noise and double counting or T-wave oversensing (see Figure 1). Clinical studies have demonstrated that malfunctions are usually asymptomatic and that RM is superior to a standard strategy for early detection.15–18 Impact of RM on device longevity is a matter of debate. Concerns have been raised about the potential negative impact of monitoring itself on battery drain. On the other hand, continuous monitoring may allow device programming optimisation with reduction of battery drain. Reduction of capacitor charges by 75 % in implantable cardioverter defibrillator (ICD) patients randomised to RM has been demonstrated in the Effectiveness and Cost of ICD Follow-up Schedule with Telecardiology (ECOST) trial with a potential major impact on battery longevity.19
Disease Management
Atrial Fibrillation
Expected benefits of RM in patients with CIED and atrial fibrillation (AF) are mainly represented by early arrhythmia detection and patient continuous monitoring. Early detection of AF may induce prompt clinical reaction aimed at preventing severe adverse events such as stroke and heart failure.20 Continuous monitoring allows individual tailoring of patient treatment and continuous updating of therapeutic strategy. AF is very common in CIED patients even in those without any history before implant. Furthermore the majority of events are asymptomatic.21 CIEDs keep detailed information about AF episodes, including number and duration, arrhythmia recurrences and burden, mean and maximum ventricular rate and intracardiac electrogram strips (see Figure 2).22 RM allows continuous access to stored data, and alerts may be programmed for specific events. Early detection of AF by RM has been demonstrated by several trials (for instance five days versus 31 days in the TRUST trial).5 Clinical evidence for stroke risk reduction by RM is still awaited. Preliminary studies estimated that daily monitoring may reduce the two-year stroke risk by 9–18 % with an absolute reduction of 0.2–0.6 %, compared with conventional inter-visit intervals of 6–12 months.23,24 In the Comparative Followup Schedule with Home Monitoring (COMPAS) trial, stroke rate was significantly higher in the control group than in the RM group (3.3 % vs 0.8 %).8 In the recent Anticoagulation Guided by Remote Rhythm Monitoring in Patients With Implanted Cardioverter-Defibrillator and Resynchronisation Devices (IMPACT) study25 of oral anticoagulation therapy for AF guided by RM (started in the case of AF detection, stopped in the case of no recurrences), there was no difference in the outcomes of stroke or all-cause mortality between the intervention group and controls. As a matter of fact, 92 % of patients who experienced stroke in the study group were either not anticoagulated at all or had an international normalised ratio (INR) < 2 at the moment of the event.
Considering that several trials have demonstrated no temporal relationship between AF episodes and stroke it should be recommended that, once started for AF, anticoagulation is not discontinued in the absence of device-detected AF.26,27
124 |
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW |

Remote Monitoring for Follow-up of Patients with Cardiac Implantable Electronic Devices
Figure 3: Ventricular Fibrillation Appropriately Detected |
Figure 4: Multiparametric Heart Failure Monitoring |
|
and Treated at Night During Sleep |
in Cardiac Resynchronisation Therapy Patient |
|
|
|
|
Shock delivery restored sinus rhythm. The patient did not perceive the shock. Automatic transmission alerted the clinical staff who called the patient for an unscheduled visit. AR = atrial event sensed in the refractory period; AP = atrial paced event;
VS=ventricular sensed event; VP=ventricular paced event; VF = ventricular event sensed in the ventricular fibrillation detection window; VT = ventricular event sensed in the ventricular tachycardia detection window; FD = ventricular fibrillation detection; CE = charge end;
CD = charge delivery.
Ventricular Arrhythmias
The significant advantage of RM is the prompt evaluation of appropriateness of detection and efficacy of therapy delivered (see Figure 3). Wireless devices send a virtually immediate transmission for review. The physician can evaluate the episode detail on the website, including internal electrograms and marker chain. With inductive systems, patients may manually send a transmission to the service centre and inform the referring physician by phone in case of perceived shock and/or palpitations or syncope. Ability of RM to early detect ventricular tachyarrhythmias has been demonstrated by the TRUST trial5 (one day vs 36 days for ventricular fibrillation and one day vs 28 days for ventricular tachycardia). Another potential benefit of RM is prevention of inappropriate shocks and also of appropriate but unnecessary shocks. Early reprogramming after inappropriate detection and therapy modulation in the case of slow, well-tolerated arrhythmias may represent the mechanisms for reaching this goal. The ECOST trial demonstrated that RM significantly reduced the number of actual delivered shocks (-72 %), the number of charged shocks (-76 %) and the rate of inappropriate shocks (-52 %).19,28
Heart Failure
In addition to providing necessary therapies for cardiac arrhythmias and heart failure (HF), modern implantable devices also provide diagnostic information that may be useful in monitoring disease progression and in early detection of deterioration of HF, such as rest and night heart rate, heart rate variability, patient daily activity, percentage of right ventricular pacing in single and dual chamber devices, percentage of actual biventricular pacing in cardiac resynchronisation therapy (CRT) devices, intrathoracic impedance or hemodynamic sensors (see Figure 4). A periodic internal electrogram is available to check actual left ventricular capture (see Figure 5). A drop in impedance is related to pulmonary congestion and it may trigger an alert if it reaches a critical threshold. Continuous monitoring of device diagnostics may allow early identification of HF
Deterioration of heart failure is clearly documented by very low cardiac resynchronisation therapy (CRT) delivery percentage and sudden drop-in thoracic impedance (arrows). CRT = cardiac resynchronisation therapy; BIV = biventricular pacing; VAR PP = heart rate variability; Brd.A = atrial fibrillation burden; IT= thoracic impedance.
progression in the phase in which the patient is still asymptomatic, but in which filling pressures increase and sympathetic activation starts. The ultimate goal is to switch clinical reaction from a ‘reactive phase’, delivered when symptoms worsen and weight increases or when the patient has a pulmonary oedema, to a ‘proactive phase’ delivered when the patient is asymptomatic, typically two to three weeks in advance. The expected results of this strategy are prevention of hospitalisations for heart failure and of disease progression and improvement of patient quality of life (QoL). Algorithms based on impedance alone showed good sensitivity in HF early detection, on average two weeks in advance, but specificity was poor.29–32 The PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) trial demonstrated that patients with a combined HF diagnostic algorithm had a 5.5-fold higher risk of HF event within 30 days.33 RM strategies have been associated with reduced unplanned device-related or cardiac in-hospital visits and reduced emergency visits for cardiac or device-related events (Evolution of Management Strategies of Heart Failure Patients with Implantable Defibrillators [EVOLVO] trial).34 Studies are ongoing to identify a combined score from device diagnostics with the greatest sensitivity and specificity for predicting HF events. Pressure-based technologies have been introduced to improve HF monitoring. Among them, a wireless implantable pulmonary artery haemodynamic monitoring system, the CardioMEMS™ (St Jude Medical, MN, US), has been recently approved by the US Food and Drug Administration (FDA). Pulmonary arterial pressure is continuously monitored and data may be reviewed by the physicians via a RM system. In the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial35 (550 patients), patients randomised to CardioMEMS guided treatment had a 37 % significant reduction in HF hospitalisation when compared with the control group.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW |
125 |

Device Therapy
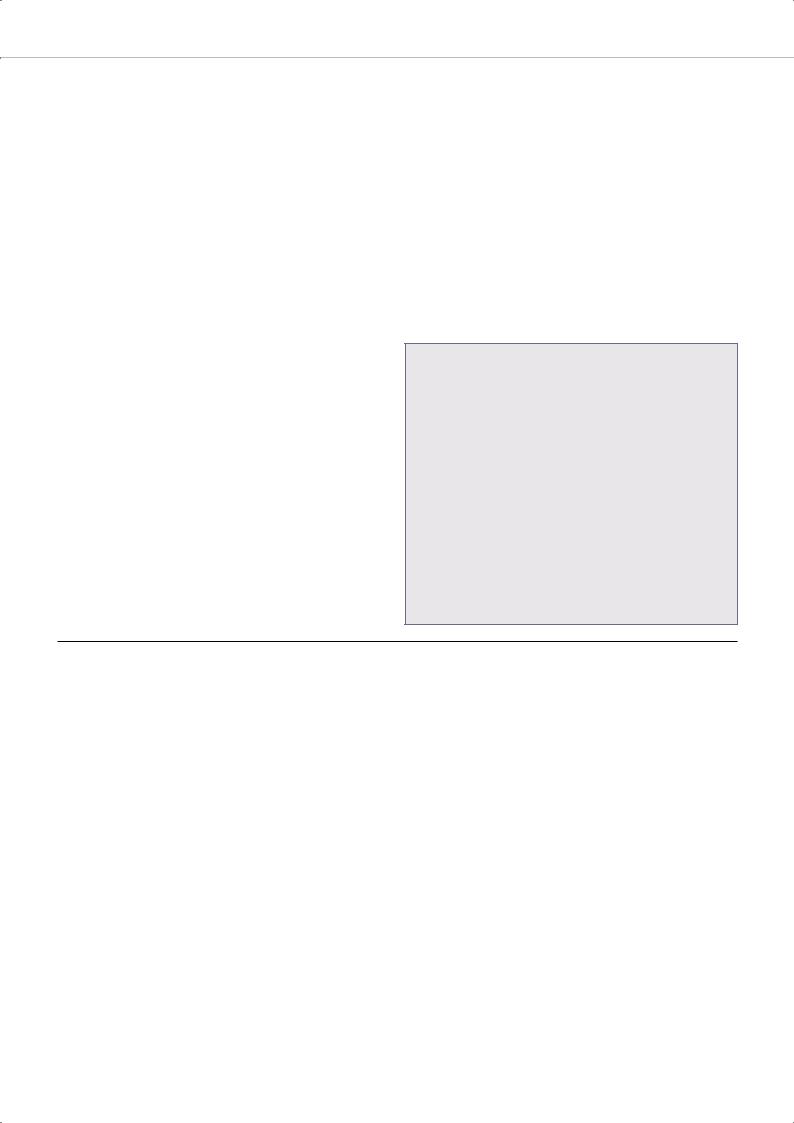
Figure 5: Periodic Internal Electrogram in a Nonresponder to Cardiac Resynchronisation Therapy
The strip shows intermittent loss of left ventricular capture in the 2nd, 3rd, 6th beat (arrows). VD = right ventricular channel; VS = left ventricular channel; VP = right ventricular pacing; VSP = left ventricular pacing.
Survival
Evidence is emerging that RM may improve survival. The ALTITUDE database (a large observational retrospective, non-randomised, post-market analysis of real-world data) enrolled about 200,000 patients,36 of whom 30 % were remotely monitored. In the RM group risk of death was reduced by 50 % both in patients with single/ dual chamber ICDs and in those with CRT defibrillators (CRT-D). In the prospective randomised IN-TIME (The Influence of ImplantBased Home Monitoring on the Clinical Management of Heart Failure Patients with an Impaired Left Ventricular Function) trial,37 after one year patients in the RM arm, further to a significant improvement in the composite clinical Packer score (mortality plus HF hospitalisations plus New York Heart Association [NYHA] class), showed a 60 % reduction in cardiovascular mortality.
Implementing Remote Monitoring in Daily Practice – The Organisational Model
In spite of the documented benefits, implementing RM in daily clinical practice is challenging and is currently offered only to a minority of potential candidates.38 Reasons for that include reluctance to accept new technology, concern for legal issues, reimbursement issues, concern for increased work burden in the transition phase, and the need to develop new organisational models. Promising results have been demonstrated by a new model based on ‘Primary Nursing’ in which each patient is assigned to a nurse responsible for continuity of care.39 The model is essentially based on a cooperative interaction between the roles of an expert reference nurse and a responsible physician with an agreed list of respective tasks and responsibilities. The model includes strict definition of workflow, early reaction, traceability, continuity of care and maintaining human relationship with the patient. Nurse duties primarily include patient training and education, website data entering, data and alert reviewing, data screening, critical case submission to the physician for clinical judgement, contacts with the patients, monitoring of patient compliance and therapy benefits. Written protocols are established in order to guide nurse reaction to findings and alerts. Physician duties include obtaining patient consent, analysis of submitted critical events and medical decisions, and communicating with general practitioners or other specialists. This model performed remarkably well in the wide Home Guide Registry which enrolled 1,650 patients.40,41 Sensitivity of RM in detecting major cardiovascular events was 84 % with a positive predictive value of 97%. RM required a median manpower less than one hour per health personnel per month for every 100 patients.
A centralised ‘hub and spoke’ model, in which one monitoring centre (hub) screened and filtered daily automatic data in pacemaker and ICD
patients from several satellite clinics (spokes), has been suggested to help smaller centres fully utilise RM technology despite limited workforce and low patient numbers that may hamper development of dedicated, experienced, single-centre RM teams.42
The use of external centralised call centres has been suggested to reduce the work burden of the hospitals and to avoid the need for on-site dedicated expert teams.43 Personnel at the call centre usually include expert technicians on duty 24 hours a day, seven days a week, with electrophysiologists available on call. Potential advantages of this model may be represented by the 24-hour service and by the possibility of following thousands of patients in a centralised station. The main limits are represented by the loss of human relationship with the patient and potentially decreased patient compliance and satisfaction. Cost and effectiveness of this strategy could be unfavourable due to call centre costs and to the risk of repetitious alerts and duplication of clinical interventions.
Patient Acceptance and Satisfaction
Several studies have shown high patient satisfaction rates, ease of use and compliance with the use of RM systems, even when manual transmission of the data in non-wireless devices is requested.44–46 In spite of some initial concerns, RM has been demonstrated to be easy to use and well accepted even for elderly people and for patients with a low level of education.47 A few patients do not accept RM mainly due to concerns about technology and about the risk of losing the human contact with nurses and physicians. Patient education is critical to overcoming their concerns.48 Poor patient compliance may complicate workflow efficiency, mainly because of missed scheduled remote transmissions or duplicate transmissions. Phonecall burden due to patient noncompliance may negatively impact on personnel work load.49 Automaticity and reliability of the remote technology used is important. In the TRUST trial, no patient assigned to RM crossed over during the study and 98 % elected to retain this follow-up mode on trial conclusion, indicating patient acceptance and confidence in follow-up with this technology.50
Legal Issues
RM changes the paradigm of face-to-face visits by gathering electronic data into a data repository that is remote from the health facility, yet readily accessed and shared with various healthcare providers involved in a patient’s care or for research or educational purposes. There are challenges to maintaining the privacy of patient health information and potential issues related to liability for RM-related services. Telemedicine is still a medical act and the patient has to be appropriately informed about the service and must sign an informed consent document. In order to ensure privacy, patients have to sign a ‘Declaration of Privacy Principles’, written according to the local privacy laws. Responsibility for the data management process lies with the device manufacturer and RM service provider (usually the same), hospitals, telecommunication technology and service providers, physicians and allied professionals and patients. In this scenario the patient is proactive and asked for their compliance with guidelines and physician’s prescriptions. Patient compliance is critical for a good result, and in some cases RM should be denied or withdrawn.
The relationship between the patients and the clinical staff is based on an atypical contract, reported in the informed consent document signed by the patient and the physician, in which general rules for patient RM are defined. In particular, scheduling for alert and transmission
126 |
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW |
Remote Monitoring for Follow-up of Patients with Cardiac Implantable Electronic Devices
revision, including the maximum reaction time, and the hospital service level agreement have to be understood and signed by the patient. The patient has to be informed that currently RM is not an emergency system, but only a tool to improve device surveillance and patient management. Clear instructions have to be given for emergency management. The technology provider is asked to respect the privacy statement and local laws, and to support the hospital centre in all aspects relating to providing patients with a RM service. Hospital duties are mainly to define and make available the facilities needed for RM.
Costs and Reimbursement Issues
Economical analyses have consistently demonstrated CIED RM to be cost effective.8,10,12,51–55 Mechanisms involved in savings include reduction of in-hospital visits, reduction of follow-up duration and physician and nurse time, and a reduction in patient costs related to travelling and missed work. Further economic benefits may come from increased device longevity, reduction of hospitalisations and prevention of clinical adverse events such as stroke. In the US since 2006 Medicare and Medicaid defined reimbursement codes for remote follow-up of pacemakers and defibrillators. Reimbursement policies for RM by healthcare systems and insurances vary widely among different countries in Europe.56 This limits adoption. Some form of reimbursement does exist in Germany, UK, Netherlands, Norway, Sweden, Finland, Denmark and Portugal. Although actual fee delivery to hospitals, doctors and manufacturers differs significantly. The fee-for-service payment approach and disease management global budget are the most common systems applied. No reimbursement exists in Switzerland, Belgium, Greece, Baltic States, Italy and Austria. This situation is rapidly evolving and soon hopefully homogeneous reimbursement rules will be established in Europe.
Current Guidelines
Since 2006, the Heart Rhythm Society (HRS)2 recommended that CIED manufacturers developed and implemented wireless and RM
technologies to early identify abnormal device behaviour and to reduce under-reporting of device malfunctions. In 2008, the HRS/ European Heart Rhythm Association (EHRA) Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices1 stated that the majority of in-person follow-up could be replaced by RM. The document suggested to maintain face-to-face visits at predischarge visits, at one-month follow-up and at least once a year. Detail on CIED RM management recommendations has been published by a joint committee of the International Society for Holter and Noninvasive Electrocardiology (ISHNE) and EHRA4 in 2012 and by some national societies.57–59 In the 2013 European Society of Cardiology Guidelines on cardiac pacing and cardiac resynchronisation therapy it was stated that “Device-based RM should be considered in order to provide earlier detection of clinical problems and technical issues” (Recommendation class IIa, level of evidence A).60 ν
Clinical Perspective
•Remote monitoring is rapidly becoming the new standard of care for patients with cardiac implantable electronic devices. The challenge is to implement remote monitoring in daily practice.
•The key to the success is from one side to invest on human resource (continuous education of personnel and development of the organisational model) and from the other to use technology progress for better interconnectivity and integration of data in the hospital electronic systems.
•Remote reprogramming which is not currently available, but technically feasible, will require great attention to patient safety prior to implementation.
•A clear reimbursement policy is mandatory to dedicate appropriate resource for a remote monitoring service.
1.Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations: developed in partnership with the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), the Heart Failure Association of ESC (HFA), and the Heart Failure Society of America (HFSA). Endorsed
by the Heart Rhythm Society, the European Heart Rhythm Association (a registered branch of the ESC), the American College of Cardiology, the American Heart Association. Europace 2008;10:707–25.
2.Carlson MD, Wilkoff BL, Maisel WH, et al. Recommendations from the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines Endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) and the International Coalition of Pacing and Electrophysiology Organizations (COPE). Heart Rhythm 2006;3:1250–73.
3.Maisel WH, Hauser RG, Hammill SC, et al. Recommendations from the Heart Rhythm Society Task Force on lead performance policies and guidelines: developed in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm 2009;6:869–85.
4.Dubner S, Auricchio A, Steinberg JS, et al. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular
implantable electronic devices (CIEDs). Europace 2012;14:278–93.
5.Varma N, Epstein A, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for ICD follow-up: the TRUST trial. Circulation 2010;122:325–32.
6.Varma N, Michalski J, Stambler B, Pavri BB; TRUST Investigators. Superiority of automatic remote monitoring compared with in-person evaluation for scheduled ICD follow-up in the TRUST trial – testing execution of the recommendations. Eur Heart J 2014;35:1345–52.
7.Crossley GH, Chen J, Choucair W, et al. on behalf of the PREFER Study Investigators. Clinical benefits of remote
versus transtelephonic monitoring of implanted pacemakers.
J Am Coll Cardiol 2009;54:2012–9.
8.Mabo P, Victor F, Bazin P, et al. A randomized trial of longterm remote monitoring of pacemaker recipients
(the COMPAS trial). Eur Heart J 2012;33:1105–11.
9.Elsner C, Sommer P, Piorkowski C, et al. A Prospective Multicenter Comparison Trial of home monitoring against regular follow-up in MADIT II. Comput Cardiol 2006;33:241–4.
10.RCrossley GH, Boyle A, Vitense H, et al. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol 2011;57:1181–9.
11.Hindricks G, Elsner C, Piorkowski C, et al. Quarterly vs. yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: results of the REFORM trial Eur Heart J. 2014;35:98–105.
12.Raatikainen MJ, Uusimaa P, van Ginneken MM, et al. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up Europace 2008;10:1145–51.
13.Ricci RP, Vicentini A, D’Onofrio A, et al. Impact of in-clinic follow-up visits in patients with implantable cardioverter defibrillators: demographic and socioeconomic analysis of the TARIFF study population. J Interv Card Electrophysiol 2013;38:101–6.
14.Kallinen LM, Hauser RG, Lee KW, et al. Failure of impedance monitoring to prevent adverse clinical events caused by fracture of a recalled high-voltage implantable cardioverter defibrillator lead. Heart Rhythm 2008;5:775–9.
15.Neuzil P, Taborsky M, Holy F,Wallbrueck K. Early automatic remote detection of combined lead insulation defect and ICD damage. Europace 2008;10:556–7.
16.Varma N. Remote monitoring for advisories: automatic early detection of silent lead failure. Pacing Clin Electrophysiol 2009;32:525–7.
17.Spencker S, Coban N, Koch L, et al. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter defibrillator patients due to lead failure.
Europace 2009;11:483–8.
18.Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance: the Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial.
Circ Arrhythm Electrophysiol 2010;3:428–36.
19.Guédon-Moreau L, Lacroix D, Sadoul N, et al. ECOST trial Investigators. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J 2013;34:605–14.
20.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614–9.
21.Orlov MV, Ghali JK, Araghi-Niknam M, et al. Asymptomatic atrial fibrillation in pacemaker recipients: incidence, progression, and determinants based on the atrial high rate trial.
Pacing Clin Electrophysiol 2007;30:404–11.
22.Varma N, Stambler B, Chung S. Detection of atrial fibrillation by implanted devices with wireless data transmission capability Pacing Clin Electrophysiol 2005;28:S133–6.
23.Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through home monitoring technology improves detection and clinical management of atrial fibrillation. Europace 2009;11:54–61.
24.Ricci RP, Morichelli L, Gargaro A, et al. Home monitoring in patients with implantable cardiac devices: is there a
potential reduction of stroke risk? Results from a computer model tested through monte carlo simulations. J Cardiovasc Electrophysiol 2009;20:1244–51.
25.Martin DT, et al. Randomized trial of anticoagulation guided by remote rhythm monitoring in patients with implanted cardioverter-defibrillator and resynchronization devices. American College of Cardiology Scientific Session, Washington, DC, March 29, 2014.
26.Glotzer TV, Daoud EG, Wyse DG, et al., on behalf of the TRENDS Investigators. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: The TRENDS Study. Circ Arrhythmia Electrophysiol 2009;2:474–80.
27.Healey JS, Connolly SJ, Gold MR, et al. ASSERT Investigators.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW |
127 |

Device Therapy
Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9.
28.Guédon-Moreau L, Kouakam C, Klug D, et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: a substudy of the ECOST trial. J Cardiovasc Electrophysiol 2014; ePub ahead of print. doi: 10.1111/ jce.12405.
29.Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005;112:84–8.
30.Conraads VM1, Tavazzi L, Santini M, et al. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J 2011;32:2266–73.
31.Catanzariti D, Lunati M, Landolina M, et al. Italian Clinical Service Optivol-CRT Group. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin Electrophysiol 2009;32:363–70.
32.van Veldhuisen DJ, Braunschweig F, Conraads V, et al. DOT-HF Investigators. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011;124:1719–26.
33.Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. PARTNERS Study Investigators. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study.
J Am Coll Cardiol 2010;55:1803–10.
34.Landolina M, Perego GB, Lunati M, et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: the evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012;125:2985–92.
35.Abraham WT1, Adamson PB, Bourge RC, et al. CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–66.
36.Saxon LA, Hayes DL, Gilliam FR, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359–67.
37.Hindricks G et al. IN-TIME: The influence of implant-based home monitoring on the clinical management of heart failure patients with an impaired left ventricular function.
ESC Annual Congress, Late breaking trials, 1 September 2013. Available at: www.escardio.org/about/press/ esccongress-2013/press-conferences/Documents/slides/ hindricks.pdf (accessed 24 June 2014).
38.Marinskis G, van Erven L, Bongiorni MG, et al. Scientific Initiative Committee, European Heart Rhythm Association. Practices of cardiac implantable electronic device follow-up: results of the European Heart Rhythm Association survey. Europace 2012;14:423–5.
39.Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and ICD patients in clinical practice. Impact on medical management and health care resource utilization. Europace 2008;10:164–70.
40.Ricci RP, Morichelli L, D’Onofrio A, et al. Effectiveness of remote monitoring of CIEDs in detection and treatment of clinical and device-related cardiovascular events in daily practice: the HomeGuide Registry. Europace 2013;15:970–7.
41.Ricci RP, Morichelli L, D’Onofrio A, et al. Manpower and outpatient clinic workload for remote monitoring of patients with cardiac implantable electronic devices: data from the HomeGuide registry. J Cardiovac Electrophysiol 2014; ePub ahead of print. doi: 10.1111/jce.12482.
42.Vogtmann T, Stiller S, Marek A, et al. Workload and usefulness of daily, centralized home monitoring for patients treated with CIEDs: results of the MoniC (Model Project Monitor Centre) prospective multicentre study. Europace 2013;15:219–26.
43.Leshem-Rubinow E, Berger M, Shacham J, et al. New realtime loop recorder diagnosis of symptomatic arrhythmia via telemedicine. Clin Cardiol 2011;34:420–5.
44.Ricci RP, Morichelli L, Quarta L, et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace 2010;12:674–9.
45.Marzegalli M, Lunati M, Landolina M, et al. Remote monitoring of CRT-ICD: the multicenter Italian CareLink evaluation–ease of use, acceptance, and organizational implications. Pacing Clin Electrophysiol 2008;31:1259–64.
46.Petersen HH, Larsen MC, Nielsen OW, et al. Patient satisfaction and suggestions for improvement of remote ICD monitoring.
J Interv Card Electrophysiol 2012;34:317–24.
47.Morichelli L, Porfili A, Quarta L, et al. Implantable cardioverter defibrillator remote monitoring is well accepted and easy
to use during long-term follow-up. J Interv Card Electrophysiol 2014, in press.
48.Morichelli L, Ricci RP. Remote monitoring of implantable devices: the European experience. Heart Rhythm 2009;6:1077–80.
49.Cronin EM, Ching EA, Varma N, et al. Remote monitoring of cardiovascular devices: a time and activity analysis.
Heart Rhythm 2012;9:1947–51.
50.Varma N, Stambler B. Patient aspects of remote home monitoring of ICDs—The TRUST Trial. Circulation 2010;123:e247.
51.Fauchier L, Sadoul N, Kouakam C, et al. Potential cost savings by telemedicine-assisted long-term care of implantable cardioverter defibrillator recipients. Pacing Clin Electrophysiol 2005;28(Suppl 1):S255–9.
52.Calò L, Gargaro A, De Ruvo E, et al. Economic impact of remote monitoring on ordinary follow-up of implantable cardioverter defibrillators as compared with conventional in-hospital visits. A single-center prospective and randomized study. J Interv Card Electrophysiol 2013;37:69–78.
53.Guédon-Moreau L, Lacroix D, Sadoul N, et al. on behalf of the ECOST trial Investigators. Costs of remote monitoring vs.
ambulatory follow-ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace 2014; Epub ahead of print. doi:10.1093/europace/euu012.
54.Zanaboni P, Landolina M, Marzegalli M, et al. Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: randomized controlled trial. J Med Internet Res 2013;15:e106.
55.Burri H, Sticherling C, Wright D, et al. Cost-consequence analysis of daily continuous remote monitoring of implantable cardiac defibrillator and resynchronization devices in the UK. Europace 2013;15:1601–8.
56.Chronaki CE, Vardas P. Remote monitoring costs, benefits, and reimbursement: a European perspective. Europace 2013;15(Suppl 1):i59–i64.
57.Ricci RP, Calcagnini G, Castro A, et al. Consensus document on remote monitoring of cardiac implantable electronic devices: technology, indications, organizational models, acceptability, responsibility, and economic issues. G Ital Cardiol 2011;12:450–67.
58.Yee R, Verma A, Beardsall M, et al. Canadian Cardiovascular Society / Canadian Heart Rhythm Society joint position statement on the use of remote monitoring for cardiovascular implantable electronic device follow-up.
Can J Cardiol. 2013;29:644–51.
59.de Cock CC, Elders J, van Hemel NM, et al. Remote monitoring and follow-up of cardiovascular implantable electronic devices in the Netherlands : An expert consensus report of the Netherlands Society of Cardiology. Neth Heart J 2012;20:53–65.
60.Brignole M, Auricchio A, Baron-Esquivias G, et al. The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2013;34:2281–329.
128 |
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW |
