
Учебники / Operative Techniques in Laryngology Rosen 2008
.pdf
■All unexplained vocal fold paralysis should be investigated with imaging studies (CT or MRI), tracing the entire RLN from skull base to upper chest.
■Parkinson’ s Disease (PD) often presents with dysphonia and vocal fold bowing and can be confused with presbylaryngis. The clinical distinction is important, as PD patients are generally poor surgical candidates, and should instead undergo voice therapy as primary treatment for their dysphonia.
Selected Bibliography
1Benninger MS, Crumley RL, Ford CN et al (1994) Evaluation and treatment of the unilateral paralyzed vocal fold. Otolaryngol Head Neck Surg 111:497–508
2Benninger MS, Gillen JB, Altman JS (1998) Changing etiology of vocal fold immobility. Laryngoscope 108:1346–1349
3Blitzer A, Brin MF, Sasaki CT et al (eds) (1992) Neurologic disorders of the larynx. Thieme, Stuttgart
4Blitzer A, Jahn AF, Keider A (1996) Semon’s law revisited: an electromyographic analysis of laryngeal synkinesis. Ann Otol Rhinol Laryngol 105:764–769
5Flowers RH, Kernodle DS (1990) Vagal mononeuritis caused by herpes simplex virus: association with unilateral vocal cord paralysis. Am J Med 1990; 88:686–688
Chapter 5 |
35 |
6Glazer HS, Aronberg DJ, Lee JKT, Sagel SS (1983) Extralaryngeal causes of vocal cord paralysis: CT evaluation. Am J Radiol 141:527–531
7Koufman JA (1995) Evaluation of laryngeal biomechanics by flexible laryngoscopy. In: Rubin JS, Sataloff RT, Korovin GS, Gould WJ (eds) Diagnosis and treatment of voice disorders. Igaku-Shoin, New York, pp 122–134
8Koufman JA, Walker FO, Joharji GM (1995) The cricothyroid muscle does not influence vocal fold position in laryngeal paralysis. Laryngoscope 105:368–372
9Koufman JA, Walker FO (1998) Laryngeal electromyography in clinical practice indications, techniques, and interpretation. Phonoscope 1:57–70
10Munin MC, Murry T, Rosen CA (2000) Laryngeal electromyography. Otolaryngol Clin North Am 33:759–770
11Netterville JL, Koriwchak MJ, Winkle M et al (1996) Vocal fold paralysis following the anterior approach to the cervical spine. Ann Otol Rhinol Laryngol 105:85–91
12Phillips TG, Green GE (1987) Left recurrent laryngeal nerve injury following internal mammary artery bypass. Ann Thoracic Surg 3:440
13Shin-ichi I kKenji K, Ken I, Oshima K (2003) Hoarseness after cardiac surgery: possible contribution of low temperature to the recurrent nerve paralysis. Laryngoscope 113:1088–1089
14Terris DJ, Arnstein DP, Nguyen HH (1992) Contemporary evaluation of unilateral vocal cord paralysis. Otolaryngol Head Neck Surg 107:84–90
15Woodson GE (1993) Configuration of the glottis in laryngeal paralysis. I: Clinical study. Laryngoscope 103:1227–1234
Chapter 6 |
|
Glottic and Subglottic Stenosis: |
6 |
Evaluation and Surgical Planning |
6.1Fundamental and Related Chapters
Please see Chaps. 9, 13, 26, 28, 29, 45, 46, and 47 for further information.
6.2Introduction
Evaluation of airway stenosis must be performed in a systematic and thorough manner to ensure accurate diagnosis and treatment planning. In the case of a patient with stridor and acute airway distress, the medical evaluation may be limited initially. However, once a secure airway is obtained, a more detailed evaluation (as outlined in this chapter) can be obtained.
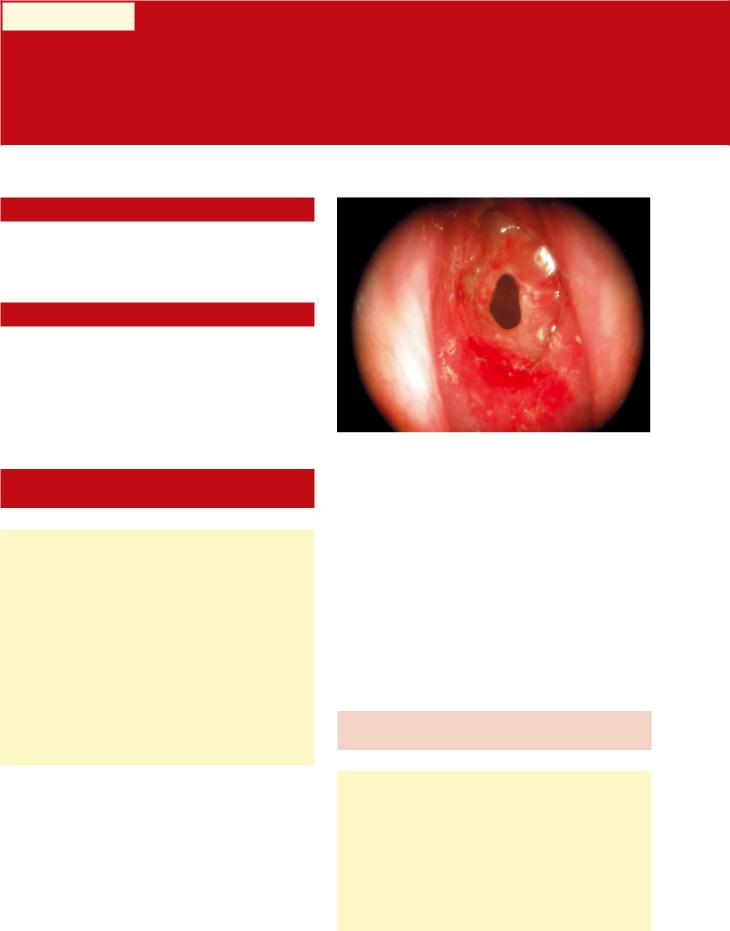
Fig. 6.1 Subglottic stenosis
6.3Etiology of Glottic and Subglottic (Laryngotracheal) Narrowing
■Prolonged endotracheal intubation
■Complications related to tracheostomy tube placement
■External laryngeal trauma
■Thermal inhalation (burn) and caustic ingestion
■Autoimmune disease
■Wegener’s granulomatosis
■Relapsing polychondritis
■Amyloidosis
■Laryngopharyngeal reflux disease
■Malignancy
■External tracheal compression (mediastinal tumor)
■Intratracheal tumor (carcinoid, metastatic tumor)
■Primary tumor of airway (e. g., chondrosarcoma of cricoid)
■Idiopathic
The vast majority of patients with glottic and subglottic airway narrowing (Fig. 6.1) are due to prolonged (at least 48–72 h or more) endotracheal intubation and complications related to tracheostomy tube placement. The endotracheal tube itself can lead to posterior glottic stenosis (PGS) from interarytenoid ulceration, pressure necrosis, and cicatricial formation in the posterior glottic space. The balloon and/or distal tip of the endotracheal tube (ETT) can likewise lead to subglottic and proximal tracheal stenosis from pressure-related effects during prolonged intubation. The risk of PGS increases markedly
after 10 days of endotracheal intubation. Additional medical factors may increase the risk of stenosis, including hypoxia, diabetes, LPR, vascular disease, localized infection, and other conditions. Tracheostomy tube placement (especially percutaneous dilational techniques) may narrow the airway through displacement of tracheal cartilage into the airway. Necrosis of tracheal cartilaginous flaps (e. g., the Björk flap) may also lead to delayed contracture and collapse of the supporting tracheal framework (see Chap. 29, Fig. 29.3). The other etiologies listed are much less common, but must be considered in non-trau- matic airway stenosis, as described below.
6.3.1Common Clinical Conditions and Associated Risk Factors
■History of prolonged mechanical ventilation
■Posterior glottic stenosis
■Subglottic/tracheal stenosis
■History of prior tracheostomy
■Tracheal collapse, typically second or third ring
■Suprastomal granulation tissue
■History of radiation to neck
■Laryngeal edema
■Glottic stenosis/fibrosis, especially in advanced T3/T4 squamous cell carcinoma (SCCa)
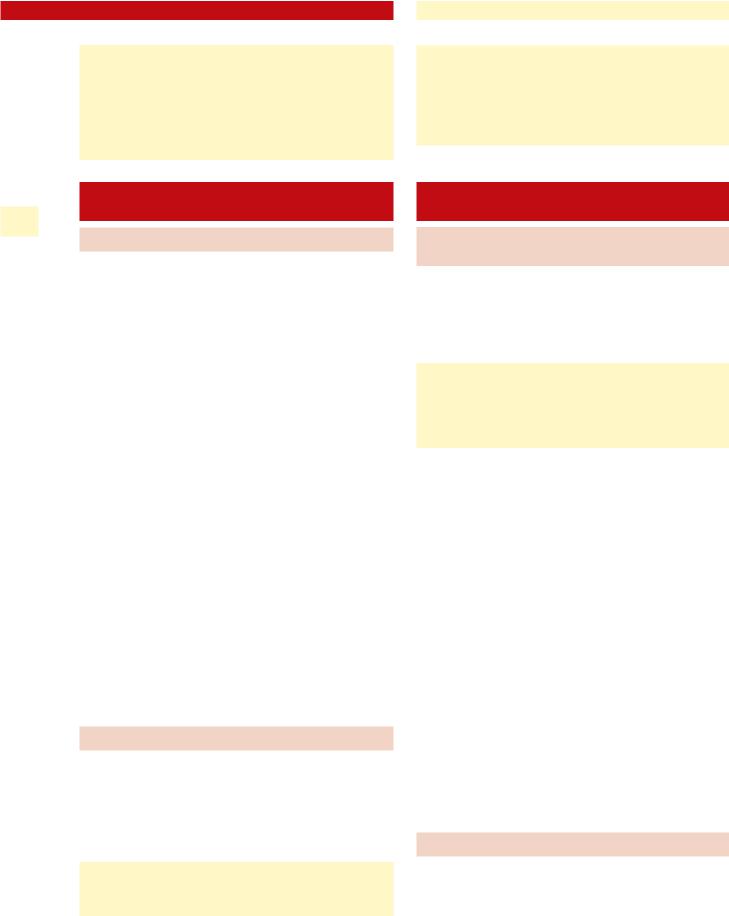
38 |
Glottic and Subglottic Stenosis |
■Nontraumatic subglottic narrowing (CRAWLS)
■Chondrosarcoma
■Relapsing polychondritis
■Amyloidosis
■Wegener’s granulomatosis
■Laryngopharyngeal reflux disease
■Sarcoidosis
6.4 Glottic and Subglottic
Stenosis: History
6
6.4.1Symptoms/Time Course
The patient with glottic or subglottic/tracheal stenosis typically presents with shortness of breath. They may have been previously misdiagnosed with asthma or “reactive airway disease,” and in many cases, they are using bronchodilators or inhaled steroids for their presumed condition. It is important to inquire specifically what level of activity the patient can tolerate before breathing difficulties are encountered (e. g., climbing up a flight of stairs, ambulating across the room, or simply at rest); this provides insight into the severity of the obstruction. In addition, one should specifically inquire whether the patient’s dyspnea is accompanied by an audible noise during inspiration.
Given the strong association between prolonged endotracheal intubation and the development of airway stenosis, one should inquire about previous intubations in the past medical history especially if they extend beyond 2–3 days. Although the risk of airway stenosis increases markedly after 10 days of intubation, it can occur occasionally with shorter exposures. In addition, caustic ingestions, thermal inhalational injuries (smoke inhalation/burns), and laryngeal trauma are also important risk factors for the development of upper airway stenosis. In many patients with airway stenosis, there is a history of tracheostomy placement and decannulation 2–3 months prior to the development of airway obstruction. In some patients, the latency to onset of airway symptoms is due to gradual maturation of scar formation in the glottis/subglottis. However, the tracheostomy can be the direct cause of the airway obstruction due to tracheal granulation tissue proliferation or cartilage resorption/collapse after decannulation.
6.4.2Medical Comorbidities
Medical comorbidities should be noted which can have a profound effect on determining if the patient is a surgical candidate for treatment of their airway stenosis. The following conditions are not absolute contraindications to surgical treatment; however, they may reduce the chances of success and/or decannulation:
■Severe restrictive or obstructive pulmonary disease (especially if oxygen dependant)
■Severe kyphoscoliosis
■Diabetes mellitus
■History of radiation therapy of the larynx
■Severe aspiration/PEG tube dependence
■Morbid obesity with severe obstructive sleep apnea (OSA)
■Autoimmune disease, especially if steroid dependent
6.5Glottic and Subglottic Stenosis: Physical Examination
6.5.1Local Anesthesia Techniques for Examination
Careful flexible laryngoscopic exam of the larynx and trachea in the clinic setting is the most important step in the evaluation of suspected glottic/subglottic stenosis. This can be achieved only if the patient’s upper airway is properly anesthetized.
These methods of anesthesia include:
■Topical lidocaine (4%) drip delivered through the side channel of the endoscope or an Abraham cannula
■Nebulized lidocaine
■Cricothyroid (or transtracheal) puncture, with instillation of 4% lidocaine
To begin the exam, the nose is anesthetized in the standard fashion for nasolaryngoscopy (lidocaine and Neosynephrine sprays). After this, the flexible endoscope is passed transnasally, and positioned over the laryngeal inlet. When properly positioned, approximately 2–3 ml of lidocaine 4% is delivered through a side channel of the scope while the patient is phonating /ē/. This may have to be repeated until the patient demonstrates little or no response to the presence of the lidocaine in the laryngeal inlet. The endoscope is then advanced through the glottis, and additional topical lidocaine is applied as needed only. An alternative is the use of an Abraham cannula, which can deliver lidocaine through a peroral technique (Fig. 6.2). The maximum recommended adult dose of lidocaine is typically 300–400 mg (7–10 ml of lidocaine 4% in a 70-kg patient).
In patients with a tracheotomy or a permanent tracheal stoma, the tracheostomy tube is removed and 2–5 ml of 4% lidocaine is dripped into the stoma. The patient’s stoma should be briefly occluded manually on anesthetic instillation so that the cough will distribute the anesthetic throughout the subglottis and trachea. With proper anesthesia, the entire laryngotracheal airway can be examined with a standard flexible endoscope in the clinic setting.
6.5.2Documentation of Examination
It is helpful to capture the flexible endoscopic airway evaluation on videotape (or digital storage device) so that a more detailed review of the airway anatomy can be carried out after the
|
Chapter 6 |
39 |
Fig. 6.2 Abraham cannula for peroral delivery of topical lidocaine to the laryngotracheal region
examination. This is especially true those patients where only a brief examination can be performed (due to poor respiratory status or inability to tolerate the procedure). In these cases, the video can be reviewed in slow motion or freeze frame to insure accuracy of the examination.
6.5.2.1Flexible Laryngoscopy/ Tracheoscopy Protocol
The following information should be obtained during the flexible laryngoscopic airway examination:
1.Vocal fold mobility testing
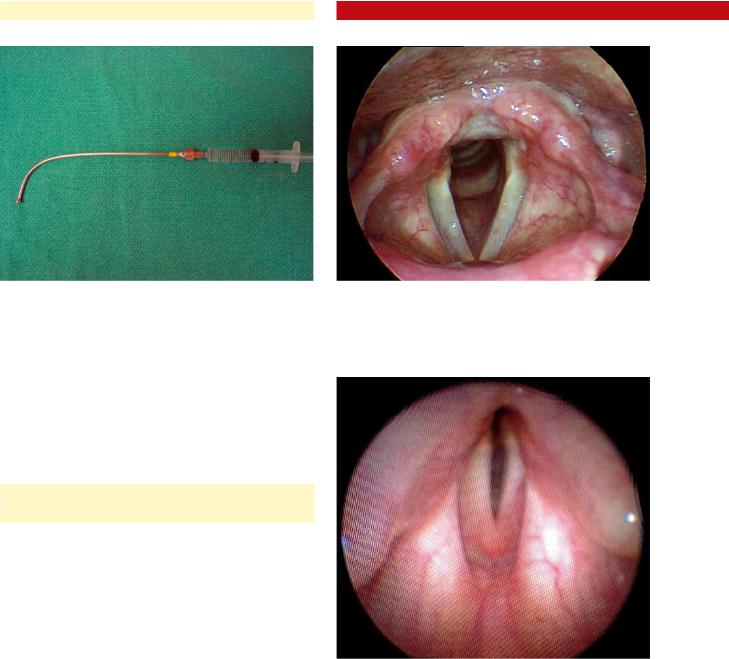
Having the patient alternate between phonating /ee/ and sniffing will test for vocal fold adduction and abduction. In general, during sniffing, maximal abduction occurs, and the glottic aperture has the general configuration of an equilateral triangle. Reduced abduction and narrowed glottic inlet can be due to posterior glottic stenosis and/or bilateral vocal fold paralysis/paresis (Figs. 6.3, 6.4). Patients with paradoxical vocal fold mobility disorder may be confused with these conditions;however,thesepatientswillgenerallyhavefullvocal fold abduction immediately after cough or other involuntary glottic closure task (see Chap. 3, “Videostroboscopy and Dynamic Voice Evaluation with Flexible Laryngoscopy”).
2.Examination of the posterior glottic space for scar
The flexible scope should be advanced into the interarytenoid space at the level of the vocal folds to evaluate for the presence of scar within the posterior glottis.
3.Scope advancement past the vocal folds, into the subglottis and trachea, including the main-stem bronchi
4.Documentation of airway measurements
If a stenosis is identified the approximate location should be noted (distance in mm distal to the vocal folds), then the length of the stenotic segment, and the estimated airway
Fig. 6.3 Normal laryngeal exam during maximal abduction (sniffing). Note the general shape of an equilateral triangle within the boundaries of the glottal aperture
Fig. 6.4 Laryngeal examination in a patient with posterior glottic stenosis during maximal abduction. Note the limited space in the posterior glottis due to interarytenoid scarring, which results in a glottic aperture of a more narrowed isosceles triangle
diameter in mm. The presence of tracheomalacia/cartilage collapse or suspected external compression of the airway should be also noted.
5.Retrograde flexible examination of the subglottic airway through the tracheal stoma (if present)
This perspective gives an unparalleled view of vocal fold mobility and posterior glottic configuration from below. The posterior glottic space can be clearly examined for scar formation.
40 |
Glottic and Subglottic Stenosis |
|
6
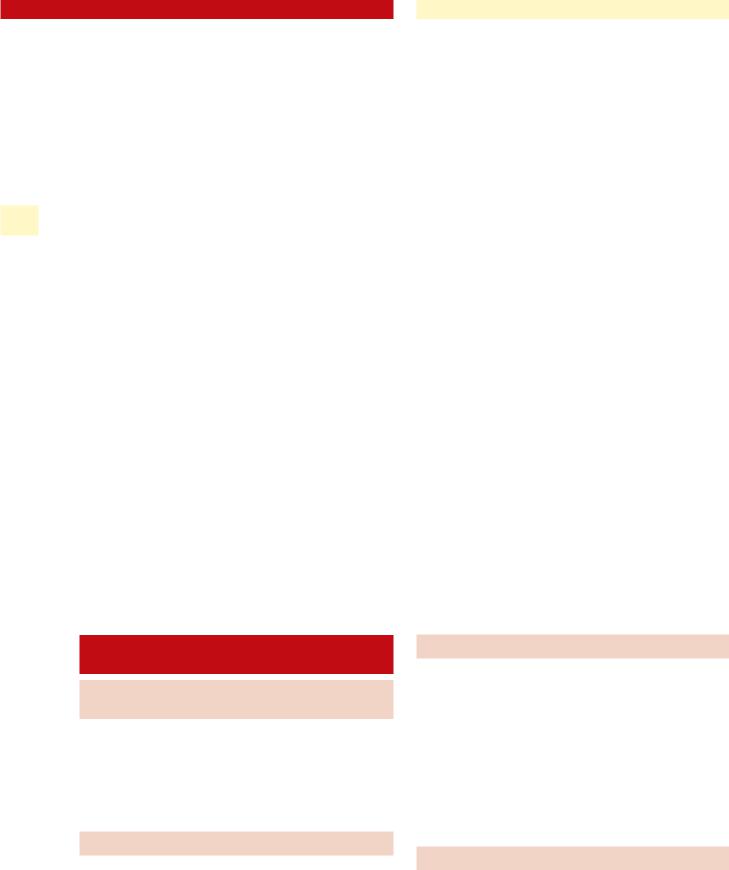
Fig. 6.5 Normal flow-volume loop |
Fig. 6.6 Flow-volume loop of patient with |
|
subglottic stenosis, demonstrating “flatten- |
|
ing” of the inspiratory limb. This is com- |
|
monly referred to as a “fixed extrathoracic |
|
obstructive pattern” |
6.6Additional Studies for the Evaluation of Glottic/Subglottic Stenosis
6.6.1Voice Evaluation
(VHI-10, Audio Recording)
Patients with glottic and subglottic stenosis often have varying degrees of dysphonia preoperatively and may develop worsening of their voice after surgery. For this reason, preoperative documentation of the voice is essential.
6.6.2Air-Flow Measures
Pulmonary function testing with a flow-volume loop can help establish the presence of upper airway obstruction. A test that is consistent with “extrathoracic airway obstruction” is typically seen in patients with glottic or subglottic/tracheal airway narrowing (Figs. 6.5, 6.6).
6.6.3Radiographic Studies
A fine-cut (1 mm) CT scan of the airway (neck and chest) with contrast is helpful in the evaluation of suspected airway obstruction. This is especially true in cases of suspected external compression or cartilage collapse (Fig. 6.7). Both of these conditions are contraindications for an endoscopic laser approach. It is important to remember that radiographic studies of the airway only provide a static view of the airway. Dynamic collapse of the airway (e. g., tracheomalacia) can only be ruled out with a flexible endoscopic examination of the entire upper airway.
6.6.4Laboratory Testing
In a small handful of patients, there is no obvious traumatic/ iatrogenic cause of the patient’s subglottic/tracheal narrowing. In these cases, one must have a high degree of suspicion for an underlying inflammatory/autoimmune, or neoplastic cause.

|
Chapter 6 |
41 |
Fig. 6.7 Computerized tomography of the trachea (axial), demonstrating collapse of the cartilaginous tracheal walls, resulting in airway narrowing. There is no evidence of intraluminal scar or soft tissue obstruction
The mnemonic for this condition (nontraumatic subglottic narrowing) is CRAWLS (see above).
In these cases, the following protocol may be used:
■c-ANCA, auto-immune profile, angiotensin-converting enzyme (ACE) level serum testing
■Biopsies of the involved tissue (histopathology and culture)
■Selective pH probe testing for LPR
6.7Glottic and Subglottic Stenosis: Surgical Planning
In most cases, the initial microlaryngoscopy/ tracheobronchoscopy is planned as a therapeutic surgery. In certain instances, however, it may be appropriate to perform an airway endoscopy in the operating room strictly for diagnostic purposes. Examples include:
■Incomplete office/radiographic evaluation of the air- way—in this case, additional information (via operative endoscopy) needs to be obtained before a definitive treatment plan can be implemented
■Suspicion of malignancy, or systemic disease—these cases should be evaluated with biopsy in the operating room. Definitive treatment may need to be delayed until histologic and/or microbiologic diagnosis is obtained, or the systemic disease is treated medically.
■Evaluation and mapping of stenosis as an aid to planning an external procedure—in this instance the patient is known to have a stenosis that is not amendable to endoscopic treatment; however, anatomic mapping of the stenosis and tracheostomy location are obtained to aid in selection of the appropriate external surgical approach. (See Chap. 29, “Subglottic Stenosis,” for details in mapping the extent of the stenosis.)
6.7.1Corrective Surgical Procedures for Glottic/Subglottic Stenosis
These procedures are listed in order from least invasive to most invasive approach.
■Endoscopic (microlaryngoscopy, laser excision, rigid dilation)
■Endoscopic with indwelling stent placement
■T-tube stent with external limb (long term)
■Intraluminal stent (short term, palliative) Dumon, Wall, Ultraflex, etc.
■External procedures
■Laryngotracheoplasty with cartilage grafting (airway expansion)
■Cricotracheal resection with primary anastomosis
In general, the least invasive procedures are attempted first (unless contraindicated), saving external procedures for those cases that fail to respond to an endoscopic approach. Airway stenting is a “middle ground” between endoscopic and external procedures; however, it is not widely practiced and requires experience to achieve consistent results. T-tube stenting is generally more successful for long-term stenosis treatment than are intraluminal stents, which have a tendency to migrate and incite granulation tissue formation. In general, intraluminal stents are not appropriate for long-term treatment of stenosis. These stents are better suited for palliative airway obstruction from metastatic tumor infiltration of the airway, and patients with terminal disease. External procedures are indicated when endoscopic treatments are contraindicated or are unsuccessful. In general, the morbidity and mortality of these procedures are significantly higher than for endoscopic treatments. Patients with significant comorbidities and advanced age may not be candidates for external stenosis treatment. Tracheostomy, although not a “corrective” procedure for airway stenosis, may be the appropriate treatment for extensive stenosis in patients with poor medical health, or when all treatments fail.
6.7.2Criteria for Endoscopic Treatment for Subglottic Stenosis
Criteria include:
■No external compression, tracheomalacia, or significant cartilage collapse
■Length of stenosis no more than 2–3 cm
■Identifiable airway lumen
■If present, tracheostomy entry point not involving/adjacent to the stenotic site
Repetitive mechanical trauma from the tracheostomy tube postoperatively has an adverse effect on healing of airway stenosis. Thus, if a tracheostomy tube is present, the stenotic region ideally should not extend down to the entry point of the tracheotomy.

42 Glottic and Subglottic Stenosis
6.7.3 Criteria for T-Tube Stenting for Subglottic Stenosis
|
|
■ Tracheotomized patients with subglottic/tracheal nar- |
|
|
rowing (from any cause) who have failed serial CO2 |
|
|
radial incisions/dilation treatment |
|
|
■ Proximal subglottic/infraglottic region free of stenosis |
|
|
■ 5- to 8-mm length of “normal” airway below vocal |
|
|
folds |
|
|
■ Accommodates proximal limb of T-tube, without |
6 |
|
|
|
impingement on vocal fold |
|
|
|
|
6.7.4 Criteria for External Treatment of Glottic/Subglottic Stenosis
Criteria include:
■Failure of endoscopic and/or T-tube stent treatments
■Extensive stenosis (no identifiable lumen, length greater than 3 cm)
■Tracheomalacia, cartilage collapse
It should be noted that the above recommendations are not absolute criteria for selecting external treatment approaches; they simply represent general guidelines. Certainly patients with lesser degrees of stenosis have failed endoscopic management, while conversely, those with more extensive stenosis have occasionally responded favorably to endoscopic treatment. The surgeon should use his/her judgment in determining suitability for endoscopic approach.
Key Points
■Laryngotracheal airway obstruction is generally caused by trauma to the upper airway from prolonged endotracheal intubation, which leads to pressure necrosis, granulation tissue, localized
infection, and cicatrix formation. The risk of airway stenosis increases markedly after 10 days of intubation.
■Tracheostomy can lead to delayed tracheal stenosis (typically 1–3 months after decannulation) and is typically due to collapse/contraction of the cartilaginous support.
■Nontraumatic subglottic narrowing should be investigated thoroughly to rule out associated inflammatory and neoplastic conditions, such as Wegener’s granulomatosis and laryngopharyngeal reflux disease.
■Physical examination of a patient with suspected laryngotracheal stenosis should include a flexible laryngoscopy and tracheoscopy (down to the carina) in the clinic setting, using topical lidocaine for endolaryngeal/tracheal anesthesia.
■Radiographic airway studies are essential if external compression is suspected, but do not replace a laryngoscopic airway evaluation.
■Corrective surgical procedures for laryngotracheal stenosis include endoscopic management (microlaryngoscopy with laser radial incisions with dilation), indwelling stent placement, and external treatments (cartilage expansion grafts vs. segmental resection and primary anastomosis).
■In patients with laryngotracheal stenosis, the least invasive surgical procedures are attempted first (unless contraindicated), reserving external procedures for those cases that fail to respond to an endoscopic approach.
■Medical comorbidities (diabetes mellitus, restrictive or obstructive pulmonary disease, and obstructive sleep apnea) may have a significant negative impact on the surgical outcome and should be carefully considered prior to undertaking these treatments.
Selected Bibliography
1Benjamin B (1993) Prolonged intubation injuries of the larynx: endoscopic diagnosis, classification, and treatment. Ann Otol Rhinol Laryngol 160(Suppl):1–15
2Amin MR, Simpson CB (2004) Office evaluation of the tracheobronchial tree. Ear Nose Throat J 83(Suppl.):10–12
3Shapshay SM, Beamis JF, Hybels RL et al (1987) Endoscopic treatment for subglottic and tracheal stenosis by radial laser incision and dilation. Ann Otol Rhinol Laryngol 96:661–664
4McCaffrey TV (1991) Management of subglottic stenosis in the adult. Ann Otol Rhinol Laryngol 100:90–94
5Gardner GM, Courey MS, Ossoff RH (1995) Operative evaluation of airway obstruction. Otolaryngol Clin North Am 28:737–750
6Lano CF Jr, Duncavage JA, Reinisch L, Ossoff RH, Courey MS, Netterville JL (1998) Laryngotracheal reconstruction in the adult: a ten-year experience. Ann Otol Rhinol Laryngol 107:92–97
Chapter 7 |
|
Nonsurgical Treatment |
7 |
of Voice Disorders |
7.1Fundamental and Related Chapters
Please see Chaps. 2, 4, 5 and 8 for further information.
7.2Introduction
Many voice problems do not require surgery if properly identified and treated. Though phonosurgical management of certain vocal pathologies is critical, many voice disorders are treated effectively by non-surgical means. This chapter gives a brief overview of several categories of voice disorders that are primarily treated without surgery.
7.3Surgical Indications and Contraindications
studies have shown that twice-a-day therapy appears to result in the highest symptom resolution. Most clinicians and studies support duration of treatment of at least 4–6 months. It takes several months for affects to be noted by the patient, so patients typically need encouragement to remain compliant with their medication.
Several controversies in the treatment of LPR include the strength of association between cough and LPR and duration of treatment. An additional point of contention is the use of histamine type 2 (H2RA) receptor antagonists in combination with PPIs. A few studies have confirmed that the H2RAs do not add any additional efficacy to treatment; however, many clinicians have noted significant improvement in LPR control with H2RAs, especially when given at night for the treatment of nocturnal acid breakthrough.
7.4Vocal Fold Granuloma
Four to 10% of otolaryngologic visits are related to gastroesophageal reflux disease-related laryngeal complaints. Up to 50% of voice disorder patients may have coexisting laryngopharyngeal reflux (LPR). LPR manifests in many ways: sore throat, globus, hoarseness, throat clearing, dysphagia, chronic cough, and postnasal drip. The diagnosis of LPR is based on patient history and laryngeal signs noted during laryngoscopy. These include edema and erythema of the larynx, pseudosulcus (infraglottic edema), Reinke’s edema, interarytenoid mucosal changes, contact ulcers, granulomas, and posterior pharyngeal mucosal cobblestoning. It has been associated as well with paradoxical vocal fold motion disorder and asthma. It has also been linked to the development of leukoplakia and potentially, laryngeal cancer. Studies have alluded to the frequent association of LPR and/or gastroesophageal reflux disease (GERD) with subglottic stenosis in adults and children. It is critical to treat LPR after any type of airway reconstruction. Symptoms can be quantified by means of the Reflux Symptom Index and findings by the Reflux Finding Score. It is felt that both the acid and pepsin contribute to the inflammation associated with LPR and/or GERD.
The gold standard in diagnosis remains the 24-h doubleprobe (esophageal and pharyngeal) pH study. With this study, a reflux event is defined as a 5 second drop in the intraluminal pH below 4.0. The standard of care for the treatment of LPR is the proton pump inhibitor (PPI), which works to irreversibly inhibit the proton pumps of the gastric parietal cell. Recent
Vocal fold granulomas (specifically nonintubation related) are notoriously recalcitrant to surgical therapy when underlying causative factors (such as LPR) are not controlled. Most vocal fold granulomas are located in the posterior third of the vocal fold either unilaterally or bilaterally. When granulomas occur postsurgically, they can occur anywhere an operative site exists. LPR plays an important role in the development of granulomas as do phonotrauma and trauma secondary to endotracheal intubation. Intubation granulomas are more common in women, presumably because their smaller larynx is more prone to trauma from the endotracheal tube. One study found that of patients with LPR, up to 75% responded to clinical treatment with PPIs; however, 21% demonstrated recurrence. The other treatment options for granulomas are voice therapy and botulinum toxin type A injection to the thyroarytenoid muscle. The latter causes a temporary paresis of the vocal fold to reduce extensive interarytenoid contact. Vocal fold granulomas also often occur (and recur) due to an underlying glottal insufficiency that may not be recognized by the treating physician. Excessive vocal fold closure pressure is applied to the arytenoids in an attempt to compensate for the glottal insufficiency, resulting in vocal fold granuloma formation or recurrence. Treatment for vocal fold granuloma due to glottal insufficiency involves vocal fold augmentation and/or medialization (see Chaps. 31, ”Vocal Fold Augmentation via Direct Laryngoscopy”; 38, “Silastic Medialization Laryngoplasty for Unilateral Vocal Fold Paralysis”; and 39, “GORE-TEX® Medialization Laryngoplasty”).

44 |
Nonsurgical Treatment of Voice Disorders |
7.5Infectious and Inflammatory Disorders
Fungal laryngitis is increasingly recognized as a cause of laryngitis. The widespread use of steroid based inhalers for the treatment of obstructive pulmonary disease has been a major contributor to the increase in fungal laryngitis incidence. Fungal laryngitis may be mistaken for leukoplakia. Clinical appearance of whitish plaques surrounded by erythematous mucosa is characteristic. Predisposing factors apart from inhaler use include radiotherapy, prolonged antibiotic use, smoking, and immunosuppression. Dysphonia may occur in
7 5–50% of patients using inhaled steroids. There appears to be a dose-dependent dysphonia in 34% of patients treated with beclomethasone dipropionate or budesonide when administered via pressured metered dose inhalers. The most common organism implicated is Candida, but the presence of Aspergillus, Blastomyces, Histoplasma, and Coccidioides has also been documented in cases of fungal laryngitis. Diagnosis is based on demonstration of fungal spores, hyphae, and/or pseudohyphae within upper epithelial layers of the laryngeal mucosa by culture or biopsy, both of which are done via laryngoscopy or office endoscopy. However, often the disease is treated clinically based on the characteristic findings. Inhalers used with a spacer decrease laryngeal deposition of the medication and can help with reduction or complete elimination of the offending agent.
Treatment of fungal laryngitis rests on removal of the offending steroid when possible and antifungal medication. If the organism persists, then treatment with an oral conazole agent for 3–4 weeks is commenced. Current standard of care, however, is use of an oral conazole medication as initial treatment especially in the immunocompromised patient.
Chemical laryngitis—specifically steroid inhaler laryngi- tis—is another common cause of dysphonia in the inhalerusing patient. Hoarseness is the most frequent local side effect of steroid inhalers. Several factors may contribute to this chemical irritation: the steroid “its preparation, the drug carrier” the type of inhaler device, mechanical irritation due to cough, inflammation of the upper airways and surrounding irritating triggers such as smoke. One study noted the following mucosal changes in patients with inhaler-related dysphonia: vascular lesions such as dilated blood vessels, capillary ectasias and varices, and “areas of thickening, irregularity, and leukoplakia.” These changes appear to improve after cessation of the steroid inhaler. In addition, some have attributed dysphonia secondary to steroid inhaler use to steroid myopathy, as both vocal folds appear atrophic and glottal closure is incomplete. Actual muscle bulk change due to steroid inhalers is controversial and not supported by scientific evidence. Some findings can overlap with those of LPR; therefore, LPR should be optimally controlled in conjunction with reduction or discontinuation, when possible, of the inhaler.
Autoimmune disorders are relatively rare but several of these have effects on the vocal folds and subglottis. Rheumatoid arthritis (RA) affects 2–3% of the adult population, and 25–53% of patients have involvement of the larynx. The main two manifestations of RA at the level of the vocal folds are cri-
coarytenoid arthritis and rheumatoid lesions of the vocal fold. Systemic treatment of RA is favored to treat rheumatoid nodules; if they persist and cause a functional voice problem, then surgery is indicated. Vocal fold hypomobility associated with cricoarytenoid (CA) arthritis has resolved in some reports with systemic treatment and possibly steroid injection into or near the CA joint. Systemic lupus erythematosus (SLE) infrequently manifests itself in the larynx but can be associated with laryngeal edema in up to 28% of patients and vocal cord paralysis in 11% of patients with SLE.
Wegener’s granulomatosis (WG) is a rare disease that involves principally three anatomical areas: the head and neck, lower respiratory tract, and the renal system. The cause of WG is unknown, but the disease is pathologically described by three findings: necrosis, granulomatous inflammation, and vasculitis. Signs associated with laryngeal involvement include wheezing or stridor, dyspnea, and dysphonia. Diagnosis is based on a blood test for the identification of antinuclear cytoplasmic antibody (ANCA) and specifically c-ANCA, which is found in 90% or more of patients with active WG. Subglottic stenosis is a major concern; the vocal folds proper are usually not involved. Systemic treatment incorporates use of corticosteroids and other immunosuppressive drugs, especially cyclophosphamide. If the stenosis is critical however, patients go on to either endoscopic or open surgical treatment, with or without tracheostomy depending on severity of the disease (see Chaps. 6, “Glottic and Subglottic Stenosis: Evaluation of Upper Airway Disorders”; 29, “Subglottic Stenosis”; 45, “Glottic and Subglottic Stenosis: Laryngotracheal Reconstruction with Grafting”; and 46, “Glottic and Subglottic Stenosis: Cricotracheal Resection with Primary Anastomosis”). The disease state should be under good medical control before performing surgical procedures for airway stenosis.
Laryngeal amyloidosis is a rare and benign idiopathic disease, which presents as a primary disease or secondary with other disease processes. It comprises 0.2–1.2% of all benign tumors. The disease is indolent and when found in the larynx, can cause slowly progressive dysphonia and dyspnea; airway symptoms in general appear to predominate. When present in a secondary form, it can be associated with multiple myeloma, medullary thyroid carcinoma, and small cell carcinoma. Amyloid deposits or lesions are described typically as “firm, nonulcerating, orange-yellow, to gray epithelial nodules.” Definitive diagnosis is based on histopathologic presence of amyloid fibrils in a twisted β-pleated sheet patter with affinity for Congo red dye. The underlying condition in the secondary form requires treatment; however, systemic treatment frequently may not eliminate the amyloid deposits. When the airway or voice is compromised, surgical intervention is warranted. Serial laser laryngoscopy is often effective at controlling symptoms. More advanced disease may require laryngofissure with partial or total laryngectomy. Primary (localized) and secondary (systemic) amyloidosis are distinguished based on physical exam (for tender bones, heart failure, hepatosplenomegaly, lymphadenopathy), blood/serum and urine testing, chest and bone radiography, abdominal subcutaneous fat aspiration, CT exam of suspicious parts of the body, and rectal biopsy.

7.6Neurologic Disorders
7.6.1Spasmodic Dysphonia
Spasmodic dysphonia (SD) is a focal dystonia characterized by vocal task specific action or intention induced spasms. Dystonias in general are disorders of central motor processing, and SD can be found in conjunction with other disorders, such as Meige’s syndrome, although typically it is isolated to the larynx. There are three classic types of SD. Adductor SD (1) comprises 80% of patients with the disorder. Abductor SD (2) and patients with both adductor and abductor activity, mixed SD (3), comprise the rest of disease population. Adductor SD is marked by a “strained-strangled” speech pattern caused by premature and excessive glottal closure, whereas abductor SD is marked by breathy speech breaks and an overall hypophonia due to inappropriate glottal opening during speech. SD typically presents in a female patient in her mid-30s, and if it has been present for some time, many patients develop compensatory changes, which may mask the true diagnosis. Patients may not demonstrate speech breaks during singing or laughing tasks, and patients find worsening of symptoms when under psychological stress. Diagnosis rests primarily on audi- tory-perceptual evaluation of connected speech supplemented by flexible nasopharyngolaryngeal examination. Diagnosis can be difficult, as patients may have associated essential tremor or actually have muscle-tension dysphonia; both disorders can cause voice breaks.
Few if any medications have been successful in ameliorating symptoms of SD. Some patients find that alcohol or benzodiazepines are helpful to reduce the stress that may be the trigger for SD. The standard of care in the treatment of SD is injection of the affected muscle(s) with botulinum toxin (BTX), which causes a temporary chemical denervation of the thyroaryte- noid–lateral cricoarytenoid muscle complex in adductor SD and the posterior cricoarytenoid muscle in abductor SD (see Chap. 35, “Botulinum Toxin Injection”). Prior to this, recurrent laryngeal nerve section was performed; however, recurrence of symptoms was typical (despite complete nerve section) and the overall voice quality worsened. Voice therapy can be used as adjunctive therapy to treat compensatory behaviors or assist in differentiating SD from muscle-tension dysphonia. Some newer surgical techniques have been developed but no long term data is available and thus are presently experimental and not validated.
7.6.2Essential Tremor
Essential tremor is the most common movement disorder, affecting 0.4–5.6% of those over age 40. However, the disease also appears to have a bimodal age distribution, with 4.6–5.3% of cases occurring in the first two decades of life. There appears to be a familial association in 17–100% of individuals transmitted in an autosomal-dominant inheritance pattern with variable penetrance. Three areas of the body may be involved to varying degrees: head, hands, and vocal tract. Essential tremor
Chapter 7 |
45 |
of the voice is seen in 12–30% of patients with essential tremor, and a head tremor in 50%. Essential tremor of the voice is marked by a regular 4- to 12-kHz frequency oscillation of the affected muscles. Several drugs have also been associated with tremor production, and Parkinson’s disease is also considered in the differential diagnosis.
Pharmacotherapy, specifically with primidone and propranolol, is employed as first-line treatment but is more effective for limb-based tremor than voice. Recently, some work has emerged concerning botulinum toxin A injections for treatment of voice tremor. The difficulty with local treatment, however, is that multiple muscles are involved in voice tremor (both intrinsic and extrinsic laryngeal musculature), so the benefit of simple thyroarytenoid–lateral cricoarytenoid muscle complex injection is not nearly comparable to benefit of botulinum toxin A seen in SD treatment. Medically refractory cases are treated with thalamotomy or deep brain stimulation (DBS); bilateral thalamotomy is associated with significant vocal side effects such as hypophonia and significant data for DBS in treatment of voice tremor is pending.
7.6.3Parkinson’s Disease
Parkinson’s disease (PD) affects nearly 1 million persons in the United States, and in severe forms, leads to considerable disability. The disease is caused by neurodegeneration within the nigrostriatal tracts of the basal ganglia, a neural center for motor control, which leads to decreased dopamine release. The hallmark clinical findings are bradykinesia, tremor, postural instability, and muscle rigidity. Phonatory effects include hypophonia, breathy dysphonia, and vocal tremor. The voice takes a monotonic quality. Many patients experience dysphagia and dysarthria. One study reported that 87% of PD patients demonstrated vocal fold bowing. The treatment of the voice component of PD involves a specialized voice therapy program, Lee Silverman Voice Treatment (LSVT), with or without vocal fold augmentation to improve glottic configuration and closure. Typically, LSVT is sufficient alone and vocal fold augmentation is not required. Treatment of PD is pharmacologic using dopamine agonists and medically refractory cases may undergo DBS or pallidotomy. No data are available currently regarding the effect of DBS on the voice in PD.
7.6.4Muscle Tension Dysphonia
Muscletension dysphonia (MTD) is a term used to describe voice disorders that are related to excessive and poorly regulated laryngeal muscle activity during speech. Many synonyms are used in clinical practice and these include hyperfunctional dysphonia, muscle misuse, and tension-fatigue syndrome to name a few. The “muscletension” descriptor has been applied to muscle contraction patterns seen on flexible laryngoscopy of the endolarynx; these are classified from types I–IV, with type I being very mild constriction with an excessive posterior glottic chink, to type IV, a concentric closure pattern of the supraglot-
