
Учебники / Operative Techniques in Laryngology Rosen 2008
.pdf
i.A CO2 laser setting typically is 4 W, superpulse with a very small spot size. An intermittent firing of 0.1-s on/0.5-s off time will also minimize collateral ther-
mal damage.
ii.A platform suction device (or a moist Cottonoid) is placed below the surgical site to protect distal structures.
iii.The vocal process location is confirmed by palpation.
iv.Incision is started just anterior to vocal process, being careful not to expose the cartilage, to avoid granulation tissue postoperatively.
v.Laser char (carbonaceous debris) should be removed by rubbing a saline soaked Cottonoid over the surgi-
cal site periodically. The CO2 laser is ineffective in a heavily charred area or bleeding operative site.
e)Extension of cordotomy
i.Once the entire vocal fold is separated from the vocal process, the cordotomy is extended into the false vocal fold tissue.
ii.Frequently, a branch of the superior laryngeal artery is encountered, and troublesome bleeding can occur.
iii.Suction and bipolar laryngeal cautery are effective in stopping the bleeding.
iv.A complete cordotomy extends laterally 3–4 mm into the false vocal fold tissue/musculature (see Figs. 27.3, 27.5, shaded portion no. 1).
v.Confirmation of complete cordotomy is achieved via endoscopic evaluation with a 0 and/or 30° telescope, confirming that the cordotomy site is flush with the lateral subglottic wall.
Chapter 27 |
169 |
vi.The residual vocal fold will retract anteriorly and appears shortened (Fig 27.4).
vii.The degree of lateral extension of the cordotomy can be adjusted based on (1) tissue response to the initial cordotomy and (2) the amount of airway improvement needed by the patient.
f)Application of LTA
i.4% lidocaine is sprayed on the vocal folds/trachea to minimize laryngospasm postoperatively.
g)Application of mitomycin C (optional)
i.Topical mitomycin C is placed (typically 0.4 mg/ml) via a soaked pledget for 5 min.
3.Medial arytenoidectomy
a)Placement of laser laryngoscope
i.Place laser laryngoscope (with built-in suction) to allow exposure of the posterior membranous vocal fold, arytenoid cartilage and posterior glottic space on the intended side of the surgical procedure.
b)Laser safety precautions
i.All laser safety precautions should be put into place prior to starting the use of the laser (see Chap. 13, “Principles of Laser Microlaryngoscopy”).
c)Laser incision
i.The CO2 laser setting should involve a small spot size (0.25–0.4 μm) at a setting of 2–4 W, super-pulse mode, and used to obliterate the medial-most portion of the arytenoid cartilage for approximately 2–3 mm in width.
ii.The anterior–posterior dimensions of this area of obliteration should be posterior to the tip of the vocal process preserving all or most of the vocal process.
iii.The area of the obliteration should not extend to the posterior arytenoid tissue and should spare adjacent mucosa in the intra-arytenoid area (Fig. 27.5, shaded portion no. 2).
iv.Titration of the amount of arytenoid cartilage that is obliterated is based on the amount of airway im-
Fig. 27.3 Lateral extent of transverse cordotomy at both the level of true and false vocal fold
Fig. 27.4 Surgical result immediately after a right posterior transverse cordotomy; note how the residual vocal fold retracts anteriorly and appears very thick and shortened

170 |
Bilateral Vocal Fold Paralysis |
|
provement that is required by the patient and tissue response after the initial aspect of the medial arytenoidectomy.
v.This is a clinical judgment and should initially be done in a very conservative fashion with an expectation that some patients may require repeat surgery to further enlarge the posterior glottic airway to a satisfactory level. If adequate surgical enlargement of the posterior glottic airway is not obtained with a conservative medial arytenoidectomy, then further arytenoid lateral to the initial defect can be removed all the way to the lateral aspect of the cricoid ring resulting in a total arytenoidectomy (see below).
vi.To further improve the posterior glottic airway, the area of ablation can be taken anteriorly to include the vocal process and a partial posterior cordectomy to the level of the lateral ventricle (see Fig. 27.5, shaded portion no. 3).
vii.Remove all laser char from the operative site with suction and moist cotton pledget.
viii.Obtain hemostasis with epinephrine-soaked (1– 10,000 concentration) pledget.
ix.Apply mitomycin C to the operative site (0.4 mg/ml
for 5 min) (optional). |
Fig. 27.5 Diagram of different degrees of arytenoid removal (medial, |
x.Spray the endolarynx with 4% plain lidocaine. total) compared to transverse cordotomy (shaded area no. 1). Laser
xi.Suction esophagus and stomach with oral gastric ablation of the medial arytenoid for medial arytenoidectomy is shown
tube placement. |
in shaded area no. 2. Laser ablation of total arytenoidectomy is shaded |
4. Total arytenoidectomy |
area no. 3 |
a)Follow the preparatory steps listed above for medial arytenoidectomy.
b)Continuous CO2 laser ablation of arytenoid tissue until the operative defect is flush with the walls of the cricoid ring, both posteriorly and laterally. Tissue removal posteriorly should not remove any interarytenoid mucosa. Evaluation of this goal can be done by:
i.Placement of a curved elevator on the lateral aspect of the subglottis and then slowly drawing the instrument in a cephalad direction feeling for a glottic level “overhang” of arytenoid tissue. If there is an “overhang,” additional arytenoid tissue can be removed (Fig. 27.6).
ii.In addition, the endoscopic evaluation of the posterior glottic airway with a 70° telescope, can identify if there is any residual arytenoid overhang that needs further laser ablation to maximize the glottic airway to complete the total arytenoidectomy procedure
5.Endo-extralarnygeal suture lateralization (based on the technique of Lichtenberger)
a)Special consideration
i.This procedure is best suited as a temporizing measure for airway improvement in early BVFP cases, ideally in the first 2 months after onset.
b)Indications:
27 |
|
i. |
Early, symptomatic BVFP (first 2 months) with un- |
|
|
|
|
certain prognosis for recovery |
|
|
|
|
|
|
|
c) |
Contraindications |
|
|
|
|
i. |
Recent trauma to the posterior glottis from indwell- |
|
|
|
|
ing endotracheal tube |
|
|
|
ii. |
Indwelling tracheostomy tube |
|
|
d) Procedure |
Fig. 27.6 Palpation of residual arytenoid overhang |
||

i.Suspension laryngoscopy is performed (jet ventilation is initiated or a small 5.0 or 5.5 endotracheal tube can be used).
ii.The skin overlying the neck on the side of the proposed suture lateralization is prepped and draped in a sterile fashion.
iii.The most medialized vocal fold is selected in this procedure. An endo-extralaryngeal needle carrier device (Richard Wolf; Fig 27.7) is loaded with a 2.0 or 0 Prolene suture. Under microscopic or telescopic visualization, the needle is positioned below the posterior vocal fold at a point just anterior to the vocal process (Fig 27.8). Using the carrier device, the needle is pushed through the larynx until the tip of the needle appears externally through the skin of the neck. The needle is grasped, and the suture is advanced through the skin and temporarily secured with a clamp (Fig 27.9).
iv.The proximal end of the same Prolene suture is then threaded through the free needle, which is designed to be used with the endo-extralaryngeal needle carrier. The procedure as in step (iii) is performed at the same location of the posterior glottis, this time at a level slightly superior to the true vocal fold (Fig 27.10). The needle is again advanced externally through the skin of the neck (Fig 27.11). A second lateralization suture is placed in a similar fashion, 1–2 mm anterior to the first suture.
v.Traction is now placed on the two sutures to create lateralization of the posterior vocal fold and expansion of the static airway dimensions (Fig 27.12).
vi.A 2-cm horizontal incision is made in the neck. The sutures are then pulled deep to the skin incision. The two ends of the suture are then tied with a surgeon’s knot over the sternohyoid muscle, using a silicone button as an anchoring point (Fig 27.13).
vii.The skin incision is closed in a standard fashion.
A permanent version of this surgery can be performed by combining this suture lateralization technique with an ipsilateral partial submucosal laser resection of the TA/LCA complex and/or a partial arytenoidectomy. This technique is illustrated in Chap. 28, (“Posterior Glottic Stenosis”).
27.6Postoperative Care and Complications
Chapter 27 |
171 |
Fig. 27.7 Endo-extralaryngeal needle carrier device (Richard Wolf)
Fig. 27.8 Infraglottic placement of suture just below the level of the vocal process
Care immediately postoperatively includes:
■Twenty-four hour observation in a monitored setting may be indicated, although these procedures can be performed on an out-patient basis, especially if a stable tracheotomy is present.
■Voice rest is not essential.
■Proton-pump inhibitor(s)
■Pain medications
■Corticosteroid taper
■Antibiotics, as per the surgeon’s discretion
Fig. 27.9 The suture is grasped by an assistant and pulled through the skin

172 |
Bilateral Vocal Fold Paralysis |
|
Fig. 27.10 The same initial suture is now placed above the vocal fold (through the ventricle) at the region of the vocal process
Fig. 27.12 After completion of the suture lateralization. Note lateralization of the vocal fold with two sutures slightly anterior to the vocal process
27 During the (expected) postoperative course, the patient will experience significant worsening of the voice, which will improve over a 2- to 3-month period and then stabilize. Followup at 2–3 week intervals for reexamination and reassurance is important during the healing phase. The expected final result
Fig. 27.11 One completed lateralization suture. This sequence will be repeated once more, slightly anterior to the previous suture placement
Fig. 27.13 The two sutures are brought deep to the skin through a separate incision and tied over the strap muscles, using a silicone button
is a small posterior glottal notch in the case of posterior transverse cordotomy (Fig. 27.14). Although the notch may appear to have only increased the airway 2–3 mm, this results in significant improvement in the patient’s airway, and only mild worsening of the patient’s voice.
Complications related to posterior transverse cordotomy, medial, and total arytenoidectomy:
1.Granuloma formation
a)Granuloma formation at the operative site is not uncommon, and should be treated by maximizing antireflux medication.
b)The granuloma tend to resolve over time, but may need to be excised if still present 3–4 months after surgery.
c)These granulomas may also cause return of airway symptoms, and must be monitored carefully.

|
Chapter 27 |
173 |
Fig. 27.14 Long-term postoperative result after a right transverse cordotomy
2.Excessive scar tissue obliterating operative site
a)Occasionally, the operative site heals completely, without the characteristic “notch.”
b)The operation can be repeated on the same side with reapplication of mitomycin C.
c)It is quite rare to need additional surgery after a second surgical procedure.
Complications related to suture lateralization include:
a)Trauma to the posterior vocal fold from excessive tension on the lateralization suture.
b)The suture may “cut into” the vocal fold, separating the muscular vocal fold from the vocal process.
c)This complication can be avoided by:
i.Not operating on vocal folds after “fresh” ETT trauma
ii.Placing the first suture anterior to the vocal process, thus avoiding the temptation to lateralize the vocal process/arytenoid tissue. The sutures must be removed promptly if significant vocal fold trauma is present.
Key Points
■Bilateral vocal fold paralysis should be differentiated from posterior glottic stenosis, even though static glottic enlargement procedures such as medial arytenoidectomy, posterior transverse cordotomy and total arytenoidectomy are often helpful for both conditions.
■Patients undergoing glottic enlargement procedures for bilateral vocal fold paralysis must be counseled regarding the exchange of improved airway for decreased voice quality and volume.
■A variety of surgical procedures are available for treatment of bilateral vocal fold paralysis. The most conservative, limited procedure should be selected initially, and then further surgery and more extensive surgery can be tailored to the patient’s airway and voice needs.
■Pre-, intraand post-operative angled telescopic (30 and 70°) evaluation of the posterior glottic airway is an essential aspect of surgery for bilateral vocal fold paralysis.
■All laser char should be removed from the operative site at the end of the surgical procedure to minimize post-operative granulation tissue formation.
■Post-operative reflux treatment should be implemented to reduce post-operative granulation formation.
Selected Bibliography
1Lichtenberger G, Toohill RJ (1997) Technique of endo-extrala- ryngeal suture lateralization for bilateral abductor vocal cord paralysis. Laryngoscope 107:1281–1283
2Bosley B, Rosen CA, Simpson CB, McMullin BT, GartnerSchmidt JL (2005) Medial arytenoidectomy versus transverse cordotomy as a treatment for bilateral vocal fold paralysis. Annals of Otology, Rhinology & Laryngology 114:922–926
3Hillel AD, Benninger M, Blitzer A et al (1999) Evaluation and management of bilateral vocal cord immobility. Otolaryngol Head Neck Surg 121:760–765
4Dray TG, Robinson LR, Hillel AD (1999) Idiopathic bilateral vocal fold weakness. Laryngoscope 109:995–1001
5Crumley RL (1993) Endoscopic laser medial arytenoidectomy for airway management in bilateral laryngeal paralysis. Ann Otol Rhinol Laryngol 102:81–84
6Dennis DP, Kashima H (1980) Carbon dioxide laser posterior cordectomy for treatment of bilateral vocal cord paralysis. Ann Otol Rhinol Laryngol 98:930–934

Chapter 28 |
|
Posterior Glottic Stenosis: |
28 |
Endoscopic Approach |
28.1Fundamental and Related Chapters
Please see Chaps. 6, 9, 10, 13, 27, and 29 for further information.
28.2Diagnostic Characteristics and Differential Diagnosis
Posterior glottic stenosis (PGS) presents as progressive airway obstruction, which develops 4–8 weeks after extubation from a period of extended mechanical ventilation (Fig. 28.1). PGS has been reported as a complication that can occur after intubation times as short as 4 days and has been linked to LPR. Often the patient complains of dysphonia after extubation. Examination will frequently reveal granulation tissue in the area of the arytenoid cartilage or over the interarytenoid cleft. This tissue prevents vocal fold approximation for voice production, and the voice is breathy or whispered. The granulation tissue itself can grow to obstruct the glottis. When this occurs, the patient is often seen by the otolaryngologist. Prompt evaluation, diagnosis, and debridement of the granulation tissue can be associated with reduction in mature scar tissue formation and lessens the overall risk of the stenosis of the airway. Frequently, however, patients are not seen acutely and as the granulation tissue resolves, mature scar tissue develops which impairs vocal fold
Fig. 28.1 Posterior glottic stenosis
mobility and leads to “mature” PGS. The diagnosis is often complicated by the presence of a tracheotomy, which increases the bacterial count in the tracheobronchial tree and may exacerbate problems with granulation tissue development. Since the patient breathes through the tracheotomy, the effect of the granulation tissue obstructing the airway goes unnoticed until it has had a chance to mature and form a scar contracture. This process typically occurs over 4–8 weeks.
Even minimal injury to the mucosa over the cricoarytenoid (CA) joint can be associated with loss of cricoarytenoid joint function for vocal fold abduction. In an animal model, laser depithelialization over the CA joint was associated with a 25% reduction in vocal fold abduction after healing. Deeper injuries were associated with a greater reduction in motion and injuries into the cricoarytenoid joint capsule were associated with fusion of the arytenoids to the cricoid.
The differential diagnosis of posterior glottic stenosis is:
■Bilateral vocal fold paralysis
■Cricoarytenoid joint ankylosis (e. g., autoimmune from rheumatoid arthritis)
■Interarytenoid synchiae
Severe injury with erosion of the CA joint from pressure due to prolonged intubation can lead to CA joint fusion. This is usually noted in the endoscopic exam, which reveals a normal posterior glottis associated with CA joint ankylosis. Often this finding can be appreciated on careful flexible laryngoscopy examination in the office under topical anesthesia. In the PGS patient, there is also a history of relatively recent intubation.
Laryngeal electromyography (EMG) can be used to distinguish PGS from immobility due to previous bilateral neurological injury or bilateral paralysis (see Chap. 2, “Principles of Clinical Evaluation for Voice Disorders”). In PGS, the EMG activity of the thyroarytenoid–lateral cricoarytenoid muscle complex will be normal, while in bilateral paralysis, even of chronic origin, there will be evidence of old neurological injury with partial recovery. EMG activity in bilateral paralysis will show reduced interference pattern often with reduced recruitment and large polyphasic motor unit potentials. Frequently, there will be active recruitment of additional motor units with voluntary activity, but this will not be normal in amount and the amplitude of the individual potentials will be increased.
Severe scarring with CA joint fixation can be distinguished from loss of mobility due to mucosal scar contraction only via an endoscopic examination and exploration. Therefore, the initial management strategies in all patients with suspected PGS should include diagnostic and staging endoscopy, with planned palpation and potential mucosal flap elevation (see

176 |
Posterior Glottic Stenosis: Endoscopic Approach |
also Chap. 27, “Bilateral Vocal Fold Paralysis”). If mucosal scar contraction is the sole reason for loss of vocal fold abduction, then mucosal flap elevation will be associated with at least temporary improvement in vocal fold abduction and airway. The patient will notice an immediate improvement in their ability to breathe in the recovery room. If this improvement does not occur, then it is unlikely that restoration of active CA joint function will be achieved, and management then needs to proceed to either (1) ablative endoscopic procedures such as posterior transverse cordotomy, partial arytenoidectomy, total arytenoidectomy, suture lateralization or (2) open approaches with posterior glottic grafting (see Chaps. 27 “Bilateral Vocal Fold Paralysis” and 46, “Glottic and Subglottic Stenosis: Cricotracheal Resection with Primary Anastomosis”).
28.3Surgical Indications and Contraindications
Indications for surgery include:
■Airway obstruction due to PGS
■Patient desire for tracheotomy decannulation
Relative contraindications include:
■Presence of aspiration
■Compromised pulmonary status
■Diabetes (more true for open procedures than endoscopic)
■Previous radiation therapy
■Unrealistic patient expectations (improvement in both airway and voice)
■Uncontrolled laryngopharyngeal reflux
28.4Surgical Equipment
Equipment needed for surgery includes:
■Standard laser microlaryngoscopy set (Chap. 13)
■Mitomycin C (0.4 mg/ml)
28.5Surgical Procedure
1.Intubate with laser safe endotracheal tube through existing tracheotomy, perform new tracheotomy, or expose larynx with suspension laryngoscope and commence jet ventilation (see Chap. 13,” Principles of Laser Microlaryngoscopy”).
2.Suspension laryngoscope details
The procedure is begun by obtaining exposure with the
28 |
largest possible laryngoscope. If the patient does not have |
|
a tracheotomy, then jet ventilation can be used to support |
respiration.
a)Posterior commissure exposure is usually obtained without the need for anterior counter pressure.
b)To help spread the vocal folds apart, it may be beneficial to insert the tip of the laryngoscope through the vocal folds. This needs to be done with extreme caution or not at all if the patient does not have a tracheotomy.
c)If the patient does not have a tracheotomy, then inserting the tip of the laryngoscope through the vocal fold or over manipulation may cause postoperative edema requiring tracheotomy. Therefore, in patients without tracheotomy, manipulation, even palpation of uninvolved tissue needs to be minimized.
d)After exposure is obtained, the posterior commissure is examined with 0, 30, and 70° angles telescopes. The mucosal integrity in terms of granulation tissue and scarring is assessed.
3.Visualize the operative field with the binocular operating microscope.
a)High magnification will help to evaluate mucosal integrity.
4.Assess passive cricoarytenoid (CA) joint mobility (see Chap. 27, “Bilateral Vocal Fold Paralysis”).
a)Palpate the arytenoids.
b)Pushing lightly on the laryngeal surface of the mid body of the arytenoid should result in translocation or lateralization of the ipsilateral vocal process and vocal fold (see Chap. 27, Figs. 27.1 and 27.2).
c)If joint mobility is impaired, then this maneuver will result in minimal vocal process displacement, and the entire larynx will move.
d)After assessment of the posterior commissure mucosa and passive CA joint mobility, decisions regarding intervention can be made.
5.Surgical options
a)Interarytenoid synchiae
i.If a bridge of mucosa between the arytenoids is identified, then this should be excised and removed (Fig. 28.2).
ii.Mitomycin C may be applied to the raw surfaces to reduce the risk of reformation of the scar band.
iii.If this procedure results in restoration of passive mobility, then the procedure is likely to be successful, and the case should be terminated.
iv.Approximately 50% of patients with an interarytenoid synchiae will regain mobility after this type of intervention. If mobility is not restored, then it is likely that injury process has resulted in exposure of the cricoarytenoid joint, with remodeling and possible fusion of the arytenoid to the cricoid. Thus, a glottic enlargement procedure will be needed such as a posterior transverse cordotomy (PTC) or medical arytenoidectomy (MA) (see Chap. 27), or permanent suture lateralization as described in this chapter.
b)Posterior scar—microtrap-door flap
i.Through palpation of the CA joints, the joint with the best mobility should be identified.
ii.The laser or a knife is used to make an incision in the mucosa over the contralateral arytenoid starting

Fig. 28.2 Interarytenoid synchiae, with dashed line indicating surgical plane of division
Fig. 28.4 Ablation of posterior glottic scar, with CO2 laser
near the vocal process, extending over the body and into the interarytenoid cleft over the interarytenoidius muscle (Fig. 28.3).
iii.Scissors or the CO2 laser is used to separate a flap of epithelium and submucosal tissue from the underlying scar.
Chapter 28 |
177 |
Fig. 28.3 Initial incision for microtrap-door flap
Fig. 28.5 Draping of microtrap-door flap
iv.The underlying scar tissue is vaporized or excised (Fig. 28.4).
v.Troublesomebleedingisstoppedbyapplyingepinephrine (1:10,000) on 0.5 × 3-cm Cottonoids. These are held in place for 1–3 min until the bleeding stops.

178Posterior Glottic Stenosis: Endoscopic Approach
vi.The flap is elevated and the scar removed until mobility is restored or the limits of the dissection are reached. Often the flap is elevated over the contralateral CA joint and 4–5 mm below the vocal folds in the interarytenoid cleft.
vii.The flap is then repositioned over the ipsilateral CA joint (Fig. 28.5).
viii.Sutures may be required to hold the flap in place.
Fig. 28.6 Outline of extent of excision in permanent suture lateralization technique
ix.Mitomycin C (0.4 mg/ml) may be applied to the exposed CA joint to lessen scar tissue formation in this region.
x.If joint mobility is not obtained, then it is unlikely that the procedure will be successful, and consideration should be given to additional procedures that enlarge the posterior glottis, such as PTC, MA, total arytenoidectomy (see Chap. 27), or irreversible suture lateralization as depicted in this chapter.
These procedures are best used when attempts at restoration of joint mobility have failed and the cartilaginous glottis is relatively well preserved. If the cartilaginous portion of the arytenoid has been resorbed by the healing process, then identification of the vocal process will be difficult. Since the area is filled in with scar tissue, incision in this area will usually result in scar reformation, without significant airway improvement.
c)Permanent suture lateralization
This technique, as described by Lichtenberger, is more appropriately performed in cases of BVFP. Only in carefully selected cases, and when the surgeon has extensive experience in the endoscopic management of PGS should one attempt this treatment for PGS. That being said, this procedure, especially if performed bilaterally can be successfully used in recalcitrant cases of PGS that do not respond to other methods such as PTC and subtotal arytenoidectomy.
xi.The skin overlying the neck is prepped and draped in a sterile fashion.
28
Fig. 28.7 CO2 laser excision of lateral arytenoid and lateral vocal fold |
Fig. 28.8 After completed excision, with extension of the excisional |
tissue |
margin below the free edge of the vocal fold |
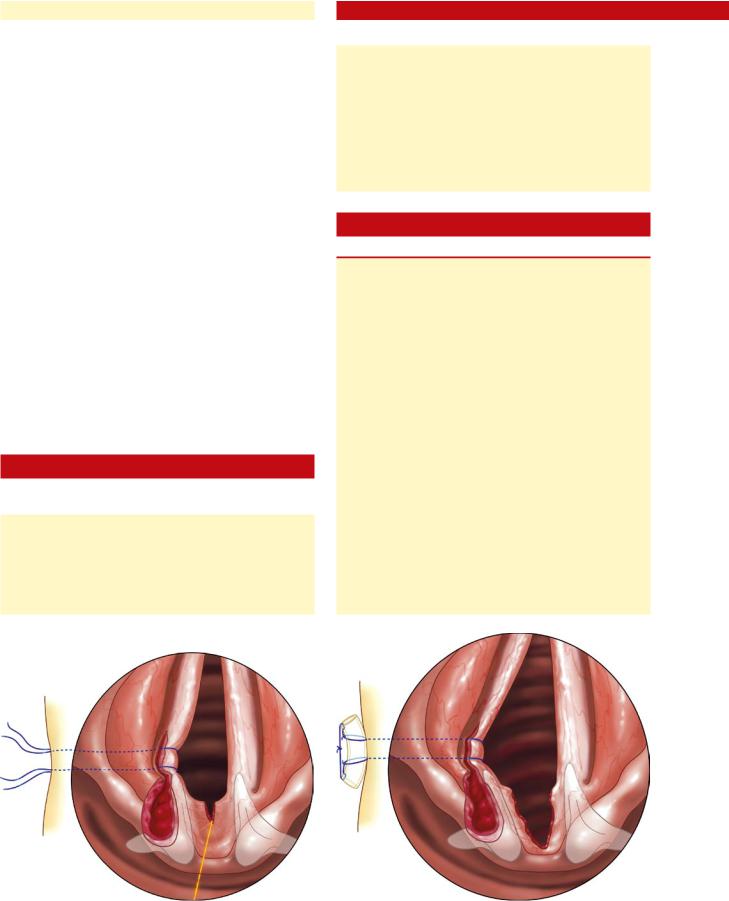
xii.As depicted in Fig. 28.6, the procedure involves a subtotal arytenoidectomy, as well as a partial removal of lateral vocal fold musculature.
xiii.Grasping the mucosa overlying the arytenoid, the
CO2 laser is used to excise the lateral aspect of the arytenoid, extending the incision anteriorly into the lateral aspect of the vocal fold for a distance of 3–4mm beyond the vocal process. The vocal process and medial aspect of the arytenoid, along with the mucosa overlying these structures are preserved (Fig. 28.7).
xiv.The excision of arytenoid and lateral vocal fold musculature should continue inferiorly such that the defect extends infraglottically below the free edge of the vocal fold (Fig. 28.8).
xv.Two sutures are used to lateralize the posterior vocal fold, as described in Chap. 27. “Bilateral Vocal Fold Paralysis”. Traction is placed on the sutures by an assistant, while the posterior glottic scar is divided with a CO2 laser (Fig. 28.9).
xvi.The sutures are then secured over a silicone button, or a modified curved plastic oral airway device
(with drill holes) (Fig. 28.10).
xvii.Often, the same process is repeated on the contralateral side to obtain maximal airway results.
28.6Postoperative Care
Relative to the postoperative course are the following:
■Voice rest is not necessary.
■The patient is encouraged to ambulate and plug their tracheotomy (if present) while awake if possible.
■Regular diet may be resumed when the effects of anesthesia are resolved
■LPR medical therapy is essential
Chapter 28 |
179 |
■The patient is reevaluated in the office at 1 month, with flexible laryngoscopy. If mobility of one or both arytenoids has been achieved, then consideration for decannulation can be undertaken.
■With the suture lateralization technique: The patient is brought to the operating room 3–4 weeks later for removal of sutures. Mitomycin C may be placed in the
posterior commissure as well as conservative removal of any granulation tissue at that time.
Key Points
■PSG needs to be distinguished from bilateral true vocal fold paralysis. Ninety-five percent of the time a history of previous prolonged intubation, followed by a 4- to 8-week time course of progressive airway obstruction, is associated with stenosis. Careful physical examination will document abnormalities of the cartilaginous glottis in over 80% of these patients.
■Laryngeal electromyography may be undertaken if the airway is safe or tracheotomy has been performed. EMG will usually show normal activity in PGS patients.
■Direct laryngoscopy with palpation can be used to confirm the suspected diagnosis.
■At the time of direct laryngoscopy, attempts to release the posterior scar band through simple excision or mucosal flaps can be undertaken.
■Surgical success is usually associated with an immediate noticeable improvement in passive mobility of one or both vocal folds. Patients will also notice improvement in respiratory status immediately after the operation.
Fig. 28.9 After suture lateralization, traction is placed on the sutures, while the posterior glottic scar is divided with the CO2 laser
Fig. 28.10 Final result, with lateralization sutures tied over a modified oral airway device external to the skin of the neck
