
Книги фарма 2 / Bertram G. Katzung-Basic & Clinical Pharmacology(9th Edition)
.pdfOral: 0.5, 1, 2 mg tablets; 2 mg/mL solution
Parenteral: 2, 4 mg/mL for IV or IM injection
Mephenytoin (Mesantoin)
Oral: 100 mg tablets
Mephobarbital (Mebaral)
Oral: 32, 50, 100 mg tablets
Oxycarbazepine (Trileptal)
Oral: 100, 300, 600 mg tablets; 60 mg/mL suspension
Pentobarbital sodium (generic, Nembutal)
Parenteral: 50 mg/mL for IV or IM injection
Phenobarbital (generic, Luminal Sodium, others)
Oral: 15, 16, 30, 60, 90, 100 mg tablets; 16 mg capsules; 15, 20 mg/5 mL elixirs Parenteral: 30, 60, 65, 130 mg/mL for IV or IM injection
Phenytoin (generic, Dilantin, others)
Oral (prompt release): 100 mg capsules; 50 mg chewable tablets; 30, 125 mg/5 mL suspension Oral extended-action: 30, 100 mg capsules
Oral slow release (Phenytek): 200, 300 mg capsules
Parenteral: 50 mg/mL for IV injection
Primidone (generic, Mysoline)
Oral: 50, 250 mg tablets; 250 mg/5 mL suspension
Tiagabine (Gabitril)
Oral: 4, 12, 16, 20 mg tablets
Topiramate (Topamax)
Oral: 25, 100, 200 mg tablets; 15, 25 mg sprinkle capsules
Trimethadione (Tridione)

Oral: 150 mg chewable tablets; 300 mg capsules; 40 mg/mL solution
Valproic acid (generic, Depakene)
Oral: 250 mg capsules; 250 mg/5 mL syrup (sodium valproate)
Oral sustained-release (Depakote): 125, 250, 500 mg tablets (as divalproex sodium)
Parenteral (Depacon): 100 mg/mL in 5 mL vial for IV injection
Katzung PHARMACOLOGY, 9e > Section V. Drugs That Act in the Central Nervous System > Chapter 24. Antiseizure Drugs* >
Chapter 25. General Anesthetics
Katzung PHARMACOLOGY, 9e > Section V. Drugs That Act in the Central Nervous System > Chapter 25. General Anesthetics >
General Anesthetics: Introduction
The physiologic state of general anesthesia typically includes analgesia, amnesia, loss of consciousness, inhibition of sensory and autonomic reflexes, and skeletal muscle relaxation. The extent to which any individual anesthetic drug can exert these effects varies with the drug, the dosage, and the clinical situation.
An ideal anesthetic drug would induce anesthesia smoothly and rapidly while allowing for prompt recovery after its administration is discontinued. The drug would also possess a wide margin of safety and be devoid of adverse effects. No single anesthetic agent is capable of achieving all of these desirable effects without some disadvantages when used alone. The modern practice of anesthesiology most commonly involves the use of combinations of intravenous and inhaled drugs, taking advantage of their individual favorable properties while attempting to minimize their potential for causing adverse reactions.
The anesthetic technique will vary depending on the proposed type of diagnostic, therapeutic, or surgical intervention. For minor procedures, so-called monitored anesthesia care or conscious sedation is used, employing oral or parenteral sedatives in conjunction with local anesthetics (see Chapter 26: Local Anesthetics). These techniques provide profound analgesia, but with retention of the patient's ability to maintain a patent airway and to respond to verbal commands. For more extensive surgical procedures, anesthesia frequently includes the use of preoperative benzodiazepines, induction of anesthesia with intravenous thiopental or propofol, and maintenance of anesthesia with a combination of inhaled and intravenous anesthetic drugs. Such protocols also often include the use of neuromuscular blocking drugs (see Chapter 27: Skeletal Muscle Relaxants).
Types of General Anesthetics
General anesthetics are usually given by inhalation or by intravenous injection.
Inhaled Anesthetics
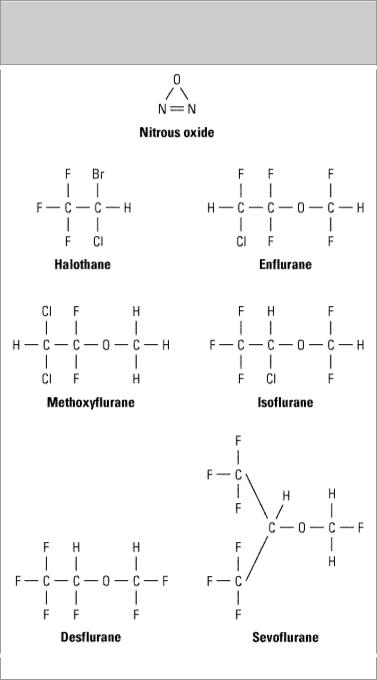
The chemical structures of the currently available inhaled anesthetics are shown in Figure 25–1. Nitrous oxide, a gas at ambient temperature and pressure, continues to be an important component of many anesthesia regimens. Halothane, enflurane, isoflurane, desflurane, sevoflurane, and methoxyflurane are volatile liquids.
Figure 25–1.
Inhaled anesthetics.
Intravenous Anesthetics
Several drugs are used intravenously, alone or in combination with other drugs, to achieve an anesthetic state (as components of balanced anesthesia) or to sedate patients in intensive care units who must be mechanically ventilated. These drugs include the following: (1) barbiturates (thiopental, methohexital); (2) benzodiazepines (midazolam, diazepam); (3) opioid analgesics (morphine, fentanyl, sufentanil, alfentanil, remifentanil); (4) propofol; (5) ketamine; and (6) miscellaneous drugs (droperidol, etomidate, dexmedetomidine). Figure 25–2 shows the structures of

commonly used intravenous anesthetics.
Figure 25–2.
Intravenous anesthetics.
Signs & Stages of Anesthesia
The traditional description of the signs and stages of anesthesia (Guedel's signs) were derived from observations of the effects of diethyl ether, which has a slow onset of central action owing to its high solubility in blood. With these signs, anesthetic effects can be divided into four stages of increasing depth of central nervous system depression.
I. Stage of Analgesia: The patient initially experiences analgesia without amnesia. Later in stage I, both analgesia and amnesia are produced.
II. Stage of Excitement: During this stage, the patient often appears to be delirious and excited but definitely is amnesic. Respiration is irregular both in volume and rate, and retching and vomiting may occur. The patient may struggle and is sometimes incontinent. For these reasons, efforts are made to limit the duration and severity of this stage, which ends with the reestablishment of regular breathing.
III. Stage of Surgical Anesthesia: This stage begins with the recurrence of regular respiration

and extends to complete cessation of spontaneous respiration. Four planes of stage III have been described in terms of changes in ocular movements, eye reflexes, and pupil size, which under specified conditions may represent signs of increasing depth of anesthesia.
IV. Stage of Medullary Depression: This stage of anesthesia includes severe depression of the vasomotor center in the medulla as well as the respiratory center. Without full circulatory and respiratory support, death rapidly ensues.
In modern anesthesia practice, the distinctive signs of each of the four stages described above are usually obscured. Reasons for this include the relatively rapid onset of action of many intravenous and inhaled anesthetics compared with ether and the fact that respiratory activity is often controlled mechanically with muscle relaxants. In addition, the practice of administering other pharmacologic agents preoperatively or intraoperatively can also alter the clinical signs of anesthesia. Atropine and glycopyrrolate are used to decrease secretions; however, they also dilate the pupils. Drugs such as tubocurarine and succinylcholine affect muscle tone, and the opioid analgesics exert depressant effects on respiration. The most reliable indication that stage III (surgical anesthesia) has been achieved is loss of the eyelash reflex and establishment of a regular respiratory pattern. The adequacy of depth of anesthesia for the specific surgical requirements is assessed by changes in respiratory and cardiovascular responses with surgical stimulation.
While vital sign monitoring remains a common method of assessing depth of anesthesia during surgery, newer techniques involving computer-assisted monitoring of cerebral function appear to offer some advantages. Automated techniques available include those based on the bispectral index (BIS), the physical state index (PSI) and the middle-latency auditory evoked potential (MLAEP), all of which are processed variables derived from established effects of anesthetics on the electroencephalogram. The application of such real-time cerebral monitoring techniques has been shown to reduce the volatile anesthetic requirement, contributing to a more rapid recovery from general anesthesia.
Katzung PHARMACOLOGY, 9e > Section V. Drugs That Act in the Central Nervous System > Chapter 25. General Anesthetics >
Inhaled Anesthetics
Pharmacokinetics
Depth of anesthesia is dependent upon the concentration of anesthetic in the central nervous system. The rate at which an effective brain concentration is reached (the rate of induction of anesthesia) depends on multiple pharmacokinetic factors that influence the uptake and distribution of the anesthetic. Certain of these factors also influence the rate of recovery from anesthesia when the anesthetic is discontinued.
Uptake & Distribution
The concentration of an individual gas in a mixture of gases is proportionate to its partial pressure or tension. These terms are often used interchangeably in discussing the various transfer processes of anesthetic gases in the body. Achievement of a brain concentration of an inhaled anesthetic adequate to cause anesthesia requires transfer of that anesthetic from the alveolar air to blood and then to brain. The rate at which a given concentration of anesthetic in the brain is reached depends on the solubility properties of the anesthetic, its concentration in the inspired air, pulmonary ventilation rate, pulmonary blood flow, and the partial pressure gradient of the anesthetic between arterial and mixed venous blood.

Solubility
One of the most important factors influencing the transfer of an anesthetic from the lungs to the arterial blood is its solubility. The blood:gas partition coefficient is a useful index of solubility and defines the relative affinity of an anesthetic for the blood compared to air. The partition coefficients for desflurane and nitrous oxide, which are not very soluble in the blood, are low (< 0.5). Halothane has a value greater than 2, and methoxyflurane, which is rarely used, has a coefficient of more than 10 (Table 25–1). When an anesthetic with low blood solubility diffuses from the lung into the arterial blood, relatively few molecules are required to raise its partial pressure, and therefore the arterial tension rises quickly (Figure 25–3, top, nitrous oxide). Conversely, for anesthetics with moderate to high solubility, more molecules dissolve before partial pressure changes significantly, and arterial tension of the gas increases less rapidly (Figure 25–3, bottom, halothane). This inverse relationship between the blood solubility of an anesthetic and the rate of rise of its tension in arterial blood is illustrated in Figure 25–4. Nitrous oxide with low solubility in blood, reaches high arterial tensions rapidly, which in turn results in rapid equilibration with the brain and fast onset of action. A rapid onset of anesthetic action is also characteristic of desflurane and sevoflurane, compounds that also have a low blood:gas partition coefficient.
Table 25–1. Properties of Inhaled Anesthetics.
|
|
|
|
|
|
|
|
|
|
|
Anesthetic |
|
Blood:Gas |
|
Brain:Blood |
|
Minimal |
Metabolism |
|
Comments |
|
|
|
Partition |
|
Partition |
|
Alveolar |
|
|
|
|
|
|
Coefficient1 |
|
Coefficient1 |
|
Conc |
|
|
|
|
|
|
|
|
|
|
(MAC) (%)2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrous oxide |
|
0.47 |
|
1.1 |
|
> 100 |
None |
|
Incomplete |
|
|
|
|
|
|
|
|
|
|
anesthetic; rapid |
|
|
|
|
|
|
|
|
|
|
onset and |
|
|
|
|
|
|
|
|
|
|
recovery |
|
Desflurane |
|
0.42 |
|
1.3 |
|
6–7 |
< 0.05% |
|
Low volatility; |
|
|
|
|
|
|
|
|
|
|
poor induction |
|
|
|
|
|
|
|
|
|
|
agent; rapid |
|
|
|
|
|
|
|
|
|
|
recovery |
|
Sevoflurane |
|
0.69 |
|
1.7 |
|
2.0 |
2–5% |
|
Rapid onset and |
|
|
|
|
|
|
|
|
(fluoride) |
|
recovery; |
|
|
|
|
|
|
|
|
|
|
unstable in soda- |
|
|
|
|
|
|
|
|
|
|
lime |
|
Isoflurane |
|
1.40 |
|
2.6 |
|
1.4 |
< 2% |
|
Medium rate of |
|
|
|
|
|
|
|
|
|
|
onset and |
|
|
|
|
|
|
|
|
|
|
recovery |
|
Enflurane |
|
1.80 |
|
1.4 |
|
1.7 |
8% |
|
Medium rate of |
|
|
|
|
|
|
|
|
|
|
onset and |
|
|
|
|
|
|
|
|
|
|
recovery |
|
Halothane |
|
2.30 |
|
2.9 |
|
0.75 |
> 40% |
|
Medium rate of |
|
|
|
|
|
|
|
|
|
|
onset and |
|
|
|
|
|
|
|
|
|
|
recovery |
|
Methoxyflurane |
|
12 |
|
2.0 |
|
0.16 |
> 70% |
|
Slow onset and |
|

|
|
|
|
(fluoride) |
recovery |
1Partition coefficients (at 37 °C) are from multiple literature sources.
2MAC is the anesthetic concentration that produces immobility in 50% of patients exposed to a noxious stimulus.
Figure 25–3.
Why induction of anesthesia is slower with more soluble anesthetic gases. In this schematic diagram, solubility in blood is represented by the relative size of the blood compartment (the more soluble, the larger the compartment). Relative partial pressures of the agents in the compartments are indicated by the degree of filling of each compartment. For a given concentration or partial pressure of the two anesthetic gases in the inspired air, it will take much longer for the blood partial pressure of the more soluble gas (halothane) to rise to the same partial pressure as in the alveoli. Since the concentration of the anesthetic agent in the brain can rise no faster than the concentration in the blood, the onset of anesthesia will be slower with halothane than with nitrous oxide.
Figure 25–4.

Tensions of three anesthetic gases in arterial blood as a function of time after beginning inhalation. Nitrous oxide is relatively insoluble (blood:gas partition coefficient = 0.47); methoxyflurane is much more soluble (coefficient = 12); and halothane is intermediate (2.3).
Anesthetic Concentration in the Inspired Air
The concentration of an inhaled anesthetic in the inspired gas mixture has direct effects on both the maximum tension that can be achieved in the alveoli and the rate of increase in its tension in arterial blood. Increases in the inspired anesthetic concentration will increase the rate of induction of anesthesia by increasing the rate of transfer into the blood according to Fick's law (see Chapter 1: Introduction). Advantage is taken of this effect in anesthetic practice with inhaled anesthetics of moderate blood solubility such as enflurane, isoflurane, and halothane, which have a relatively slow onset of anesthetic effect. For example, a 3–4% concentration of halothane may be administered initially to increase the rate of induction; this is reduced to 1–2% for maintenance when adequate anesthesia is achieved. In addition, these anesthetics are often administered in combination with a less soluble agent (eg, nitrous oxide) to reduce the time required for loss of consciousness.
Pulmonary Ventilation
The rate of rise of anesthetic gas tension in arterial blood is directly dependent on both the rate and depth of ventilation (ie, minute ventilation). The magnitude of the effect varies according to the blood:gas partition coefficient. An increase in pulmonary ventilation is accompanied by only a slight increase in arterial tension of an anesthetic with low blood solubility or low coefficient but can significantly increase tension of agents with moderate or high blood solubility (Figure 25–5). For example, a fourfold increase in ventilation rate almost doubles the arterial tension of halothane during the first 10 minutes of anesthesia but increases the arterial tension of nitrous oxide by only 15%. Therefore, hyperventilation increases the speed of induction of anesthesia with inhaled anesthetics that would normally have a slow onset. Depression of respiration by opioid analgesics will slow the onset of anesthesia of some inhaled anesthetics if ventilation is not assisted.

Figure 25–5.
Ventilation rate and arterial anesthetic tensions. Increased ventilation (8 versus 2 L/min) has a much greater effect on equilibration of halothane than nitrous oxide.
Pulmonary Blood Flow
Changes in blood flow to and from the lungs influence transfer processes of the anesthetic gases. An increase in pulmonary blood flow (increased cardiac output) slows the rate of rise in arterial tension, particularly for those anesthetics with moderate to high blood solubility. This is because increased pulmonary blood flow exposes a larger volume of blood to the anesthetic; thus, blood "capacity" increases and the anesthetic tension rises slowly. A decrease in pulmonary blood flow has the opposite effect and increases the rate of rise of arterial tension of inhaled anesthetics. In a patient with circulatory shock, the combined effects of decreased cardiac output (resulting in decreased pulmonary flow) and increased ventilation will accelerate the induction of anesthesia with halothane and isoflurane. This is not likely to occur with nitrous oxide, desflurane, or sevoflurane because of their low blood solubility.
Arteriovenous Concentration Gradient
The anesthetic concentration gradient between arterial and mixed venous blood is dependent mainly on uptake of the anesthetic by the tissues, including nonneural tissues. Depending on the rate and extent of tissue uptake, venous blood returning to the lungs may contain significantly less anesthetic than arterial blood. The greater this difference in anesthetic tensions, the more has been taken up by viscera, muscle, etc, and the more time it will take to achieve equilibrium with brain tissue. Anesthetic entry into tissues is influenced by factors similar to those that determine transfer from lung to blood, including tissue:blood partition coefficient, rates of blood flow to the tissues, and
concentration gradients.
During the induction phase of anesthesia, the tissues that exert greatest influence on the arterialvenous anesthetic concentration gradient are those which are highly perfused. These include the brain, heart, liver, kidneys, and splanchnic bed, which together receive over 75% of the resting cardiac output. In the case of anesthetics with relatively high solubility in these tissues, venous blood concentration will initially be very low, and equilibrium with arterial blood is achieved slowly.
During maintenance of anesthesia with inhaled anesthetics, these drugs may continue to be transferred between various tissues at rates dependent on solubility and blood flow. Muscle and skin, which together constitute 50% of body mass, accumulate anesthetics more slowly than the highly vascularized tissues (eg, brain), since they receive only one-fifth the blood flow of the latter groups. Although most anesthetic gases have high solubility in adipose tissues, low blood perfusion rates to these tissues delay accumulation, and equilibrium is unlikely to occur with most anesthetics during the time usually required for surgery.
Elimination
The time to recovery from inhalation anesthesia depends on the rate of elimination of anesthetics from the brain after the inspired concentration of anesthetic has been decreased. Many of the processes of anesthetic transfer during recovery are similar to those that occur during induction of anesthesia. One of the most important factors governing rate of recovery is the blood:gas partition coefficient of the anesthetic agent (see below). Other factors controlling rate of recovery include the pulmonary blood flow, the magnitude of ventilation, and the solubility of the anesthetic in the tissues. Two features of recovery, however, are quite different from what happens during induction of anesthesia. First, while transfer of an anesthetic from the lungs to blood can be enhanced by increasing its concentration in inspired air, the reverse transfer process cannot be enhanced, since the concentration in the lungs cannot be reduced below zero. Second, at the beginning of recovery, the anesthetic gas tension in different tissues may be quite variable, depending on the specific agent and the duration of anesthesia. With induction of anesthesia, the initial anesthetic tension in all tissues is zero.
Inhaled anesthetics that are relatively insoluble in blood (low blood:gas partition coefficient) and brain are eliminated at faster rates than more soluble anesthetics. The washout of nitrous oxide, desflurane, and sevoflurane occurs at a rapid rate, which leads to a more rapid recovery from their anesthetic effects compared to halothane and isoflurane. Halothane is approximately twice as soluble in brain tissue and five times more soluble in blood than nitrous oxide and desflurane; its elimination therefore takes place more slowly, and recovery from halothane anesthesia is predictably less rapid. The duration of exposure to the anesthetic can also have a marked effect on the time of recovery, especially in the case of more soluble anesthetics. Accumulation of anesthetics in tissues, including muscle, skin, and fat, increases with continuous inhalation (especially in obese patients), and blood tension may decline slowly during recovery as the anesthetic is gradually eliminated from these tissues. Thus, if exposure to the anesthetic is short, recovery may be rapid even with the more soluble agents. However, after prolonged anesthesia, recovery may be delayed even with anesthetics of moderate solubility such as isoflurane.
Clearance of inhaled anesthetics by the lungs into the expired air is the major route of their elimination from the body. However, metabolism by enzymes of the liver and other tissues may also contribute to the elimination of volatile anesthetics. For example, the elimination of halothane during recovery is more rapid than that of enflurane, which would not be predicted from their
